Physics 8 - Dallas ISD
... acquires a negative charge (Fig. B), and the charge within the sphere quickly distributes itself uniformly throughout the sphere (Fig. C). ...
... acquires a negative charge (Fig. B), and the charge within the sphere quickly distributes itself uniformly throughout the sphere (Fig. C). ...
The Quantum Numbers
... The fourth quantum number (ms) is the spin quantum number and describes the electron spin. If two negatively charged particles occupy the same orbital, how do they keep from repelling one another? It is possible the electrons spin in opposite directions and therefore, produce opposite magnetic field ...
... The fourth quantum number (ms) is the spin quantum number and describes the electron spin. If two negatively charged particles occupy the same orbital, how do they keep from repelling one another? It is possible the electrons spin in opposite directions and therefore, produce opposite magnetic field ...
Document
... The answer is b. If you answered a, you likely thought the bending was due to positively charged water. But the water, even with many ions, normally has no appreciable net charge. The interaction between the charged rod and the water stream is mainly due to the dipole nature of water molecules. H2O ...
... The answer is b. If you answered a, you likely thought the bending was due to positively charged water. But the water, even with many ions, normally has no appreciable net charge. The interaction between the charged rod and the water stream is mainly due to the dipole nature of water molecules. H2O ...
File
... _______25. In the Stern-Gerlach experiment, silver atoms were shot through a powerful magnetic field. The stream of atoms divided into two separate paths. This division would not be observed with atoms of A) Cu B) Cr C) Mg D) K E) Al ______26. The Pauli exclusion principle states that A) the velocit ...
... _______25. In the Stern-Gerlach experiment, silver atoms were shot through a powerful magnetic field. The stream of atoms divided into two separate paths. This division would not be observed with atoms of A) Cu B) Cr C) Mg D) K E) Al ______26. The Pauli exclusion principle states that A) the velocit ...
Chapter 2 – The Structure of the Atom Since the book assumes you
... nucleus and protons in the early 20th century required a model of the atom classical physics could not provide. The first real model to describe the atom with some accuracy was proposed by Neils Bohr and is the familiar electron traveling around a nucleus in circular orbits. This model employed seve ...
... nucleus and protons in the early 20th century required a model of the atom classical physics could not provide. The first real model to describe the atom with some accuracy was proposed by Neils Bohr and is the familiar electron traveling around a nucleus in circular orbits. This model employed seve ...
2012-2013 CHEMISTRY REVIEW: Organize your NOTEBOOK to
... o Scientific method and proper experimental procedures o Derived units and density calculations Chapter 3: The Building Blocks of Matter o Laws of conservation of mass, definite proportions, and multiple proportions o History of atomic theory: Dalton, Thomson, Rutherford…experiments and resulting mo ...
... o Scientific method and proper experimental procedures o Derived units and density calculations Chapter 3: The Building Blocks of Matter o Laws of conservation of mass, definite proportions, and multiple proportions o History of atomic theory: Dalton, Thomson, Rutherford…experiments and resulting mo ...
Practice problems Chapter 6.tst
... C) the darkening of photographic film when exposed to an electric field D) the production of current by silicon solar cells when exposed to sunlight E) the total reflection of light by metals giving them their typical luster ...
... C) the darkening of photographic film when exposed to an electric field D) the production of current by silicon solar cells when exposed to sunlight E) the total reflection of light by metals giving them their typical luster ...
5.62 Physical Chemistry II
... For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. ...
... For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. ...
Interaction of Radiation with Matter
... particle. Whether the interaction involves a repulsive or attractive force between the incident particle and orbital electron, the impulse and energy transfer for particles of equal mass are about the same. For positrons the annihilation radiation is generated at the end of the track. Because these ...
... particle. Whether the interaction involves a repulsive or attractive force between the incident particle and orbital electron, the impulse and energy transfer for particles of equal mass are about the same. For positrons the annihilation radiation is generated at the end of the track. Because these ...
II КУРС. ДОМАШНЯ КОНТРОЛЬНА РОБОТА № 2. Варіант № 1
... molecules,while the electrons of which the atoms are composed are yet smaller. From various experiments scientists concluded that the diameter of the average molecule is about one 125,000,000th of an inch. Since atoms compose molecules, they must be still smaller. If each atom in an orange measured ...
... molecules,while the electrons of which the atoms are composed are yet smaller. From various experiments scientists concluded that the diameter of the average molecule is about one 125,000,000th of an inch. Since atoms compose molecules, they must be still smaller. If each atom in an orange measured ...
Quantum Mechanics
... 2.35 Evidence for the electron spin was provided by the Sterrn–Gerlah experiment. Sketch and briefly describe the key features of the experiment. Explain what was observed and how this observation may be interpreted in terms of electron spin. [Adapted from University of London 2006] 2.36 (i) Write d ...
... 2.35 Evidence for the electron spin was provided by the Sterrn–Gerlah experiment. Sketch and briefly describe the key features of the experiment. Explain what was observed and how this observation may be interpreted in terms of electron spin. [Adapted from University of London 2006] 2.36 (i) Write d ...
Physics I - Rose
... E = 3.45 104 N/C, upward. EVALUATE: In both cases, the fields are of the same order of magnitude, but the values are different because the charge has been bent into different shapes. 21.78. IDENTIFY: For the acceleration (and hence the force) on Q to be upward, as indicated, the forces due to q1 a ...
... E = 3.45 104 N/C, upward. EVALUATE: In both cases, the fields are of the same order of magnitude, but the values are different because the charge has been bent into different shapes. 21.78. IDENTIFY: For the acceleration (and hence the force) on Q to be upward, as indicated, the forces due to q1 a ...
PDF only - at www.arxiv.org.
... slow and keep at T1 =(473±0.5)K for 3 hours. It means that the readings of capacitance are obtained under the condition of Cs or Hg saturated vapor pressure. Two comparable experimental curves are shown in Fig.2. From Ref.[15], the saturated pressure of Hg vapor PHg =2304.4 Pa at 473K and measuring ...
... slow and keep at T1 =(473±0.5)K for 3 hours. It means that the readings of capacitance are obtained under the condition of Cs or Hg saturated vapor pressure. Two comparable experimental curves are shown in Fig.2. From Ref.[15], the saturated pressure of Hg vapor PHg =2304.4 Pa at 473K and measuring ...
Particle-based Collision Detection
... Each model has a surface that is for the most part plain Face's adjacency information is available, i.e., there is a list of neighbor faces for each model point, or it can easily be created Adjacency information does not change during deformation, which means that bodies do not change their topology ...
... Each model has a surface that is for the most part plain Face's adjacency information is available, i.e., there is a list of neighbor faces for each model point, or it can easily be created Adjacency information does not change during deformation, which means that bodies do not change their topology ...
Precise Measurement of Time
... provid~ an excellent basis for a highpreci ion dock. Before such a clock can be built, however, there must be a suitable means for observing and counting this internal atomic periodicity. The first method for accurately measuring hyperfine frequencies was provided by molecular beam magnetic resonanc ...
... provid~ an excellent basis for a highpreci ion dock. Before such a clock can be built, however, there must be a suitable means for observing and counting this internal atomic periodicity. The first method for accurately measuring hyperfine frequencies was provided by molecular beam magnetic resonanc ...
AP Chemistry Summer Assignment - 2015
... Oxygen usually has an oxidation number of –2. Exceptions: In peroxides, such as H2O2, oxygen’s oxidation number is –1. In compounds with fluorine, such as OF2, oxygen’s oxidation number is +2. 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more electronegat ...
... Oxygen usually has an oxidation number of –2. Exceptions: In peroxides, such as H2O2, oxygen’s oxidation number is –1. In compounds with fluorine, such as OF2, oxygen’s oxidation number is +2. 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more electronegat ...
Slide 1
... •A mass spectrometer is an instrument that separates particles into their masses and records the relative proportions of these. •In a mass spectrometer (five parts): •the substance is first converted to atoms or molecules in the vapor phase. [Vaporization] •These are then turned into positive ions [ ...
... •A mass spectrometer is an instrument that separates particles into their masses and records the relative proportions of these. •In a mass spectrometer (five parts): •the substance is first converted to atoms or molecules in the vapor phase. [Vaporization] •These are then turned into positive ions [ ...
Quantum numbers
... Syntax: nlx, with x = # of electrons • Carbon: (1s2) 2s2, 2p2; Sulfur: (…), 3s2, 3p4 • Homework: write down the electron configurations of N, O, Cl why do halogens (X) form X2 in the gas phase? why do the alkali metals (Li, Na, ….) do so too? ...
... Syntax: nlx, with x = # of electrons • Carbon: (1s2) 2s2, 2p2; Sulfur: (…), 3s2, 3p4 • Homework: write down the electron configurations of N, O, Cl why do halogens (X) form X2 in the gas phase? why do the alkali metals (Li, Na, ….) do so too? ...
Quanta3 - UF Physics
... regardless of the light intensity. In classical electromagnetism, you might expect that higher intensities transfer a greater amount of energy to the electrons, thereby requiring a larger voltage to stop them. 2. The maximum kinetic energy of the emitted photoelectrons at the photocathode depends on ...
... regardless of the light intensity. In classical electromagnetism, you might expect that higher intensities transfer a greater amount of energy to the electrons, thereby requiring a larger voltage to stop them. 2. The maximum kinetic energy of the emitted photoelectrons at the photocathode depends on ...
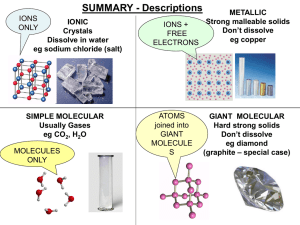
smart_materials_1 - Aldercar High School
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
Energy level - Spring-Ford Area School District
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
... of atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. • This causes difficulties because of the overlap of orbitals of different energies – follow the diagram! 2) Pauli Exclusion Principle - at most 2 electrons per orbital - different spins ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.