Electrostatics Practice Test Which one of the following represents
... much work was done on this charge? A. 30 J C. 180 J B. 160 J D. 220 J 18. A proton initially at rest is accelerated between parallel plates through a potential difference of 300 V . What is the maximum speed attained by the proton? A. 7.5 ×103m/s C. 2. 4 ×105m/s B. 1.7 ×105m/s D. 1.2 ×106 m/s ...
... much work was done on this charge? A. 30 J C. 180 J B. 160 J D. 220 J 18. A proton initially at rest is accelerated between parallel plates through a potential difference of 300 V . What is the maximum speed attained by the proton? A. 7.5 ×103m/s C. 2. 4 ×105m/s B. 1.7 ×105m/s D. 1.2 ×106 m/s ...
1.2 The Mole Concept
... • Chemists frequently attempt to determine the composition of a substance through chemical analysis • Qualitative analysis – what elements are in a compound • Quantitative analysis – calculate relative masses to determine exact composition ...
... • Chemists frequently attempt to determine the composition of a substance through chemical analysis • Qualitative analysis – what elements are in a compound • Quantitative analysis – calculate relative masses to determine exact composition ...
urbano, mariajose
... • Have specific chemical and physical properties. • Are the regions of organic molecules which are commonly chemically reactive. • Behave consistently from one organic molecule to another. • Depending upon their number and arrangement, determine unique chemical properties of organic molecules in whi ...
... • Have specific chemical and physical properties. • Are the regions of organic molecules which are commonly chemically reactive. • Behave consistently from one organic molecule to another. • Depending upon their number and arrangement, determine unique chemical properties of organic molecules in whi ...
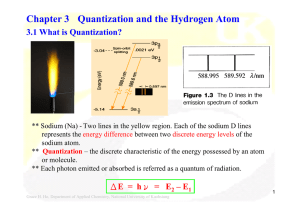
Chapter 3 Quantization and the Hydrogen Atom
... E (Eigenvalue): discrete energy levels of the system Ψ(Eigenfunction): the wave function corresponding to each eigenvalue - Schrödinger equation to obtain wave function corresponding to the known energy levels from spectroscopic measurements. - Assumed forms of wave functions to predict the energy l ...
... E (Eigenvalue): discrete energy levels of the system Ψ(Eigenfunction): the wave function corresponding to each eigenvalue - Schrödinger equation to obtain wave function corresponding to the known energy levels from spectroscopic measurements. - Assumed forms of wave functions to predict the energy l ...
Final Exam Study Guide Word document
... Understand that the kinetic theory of gases relates the absolute temperature of a gas to the average kinetic energy of its molecules or atoms. Chapter 6 Thermochemistry” Energy Flow and Chemical Change Learning Objectives: Students should be able to: ...
... Understand that the kinetic theory of gases relates the absolute temperature of a gas to the average kinetic energy of its molecules or atoms. Chapter 6 Thermochemistry” Energy Flow and Chemical Change Learning Objectives: Students should be able to: ...
Atomic Theory - New Senior Secondary Curriculum Goals
... explanation of some phenomena. For example, the pressure law states that, in a fixed mass and volume of gas, the temperature of the gas sample varies in direct proportion to its pressure. It should be noted that the pressure law (i) is not absolute – the behaviour of real gases can deviate from it; ...
... explanation of some phenomena. For example, the pressure law states that, in a fixed mass and volume of gas, the temperature of the gas sample varies in direct proportion to its pressure. It should be noted that the pressure law (i) is not absolute – the behaviour of real gases can deviate from it; ...
Atoms - York University
... beta rays – turned out to be the same as cathode rays or electrons gamma rays – light of a small wave length, something like x-rays ...
... beta rays – turned out to be the same as cathode rays or electrons gamma rays – light of a small wave length, something like x-rays ...
Thermionic emission
... •Electrons emitted by thermionic emission will soon jump back onto the atoms they came from. •However a large positive voltage can be used to attract the free electrons away from the metal that they came from. •This accelerating voltage needs to be very large often 100’s or even 1000’s of volts. ...
... •Electrons emitted by thermionic emission will soon jump back onto the atoms they came from. •However a large positive voltage can be used to attract the free electrons away from the metal that they came from. •This accelerating voltage needs to be very large often 100’s or even 1000’s of volts. ...
Electric Force Solutions
... Since both forces are attractive and follow the inverse-square law, any change in separation will affect both forces in the same way (i.e. as r increases, so does Fg and Fe , as r decreases, so does Fg and Fe). So there is no point at which the two forces could be equal. 3. Two uniformly charged sph ...
... Since both forces are attractive and follow the inverse-square law, any change in separation will affect both forces in the same way (i.e. as r increases, so does Fg and Fe , as r decreases, so does Fg and Fe). So there is no point at which the two forces could be equal. 3. Two uniformly charged sph ...
valence electrons
... & position of a particle at the same time. • In order to find position of an electron, need photon of light. • Photon “bumps” into electron, changing its position. ...
... & position of a particle at the same time. • In order to find position of an electron, need photon of light. • Photon “bumps” into electron, changing its position. ...
12.3 Assembly of distinguishable Particles
... The only function for which the above relationship is true is the logarithm. Therefore: S = k · lnW where k is the Boltzman constant with the units of entropy. ...
... The only function for which the above relationship is true is the logarithm. Therefore: S = k · lnW where k is the Boltzman constant with the units of entropy. ...
Advanced electronic bonding and how these affect molecular shapes
... electrons whirling through circular orbits. • Rather, we now know we cannot pinpoint an electron’s exact location. • This is because of the Heisenberg uncertainty principle. An electron cannot be pinpointed as the photon striking it will cause it to change momentum and position, so you will never be ...
... electrons whirling through circular orbits. • Rather, we now know we cannot pinpoint an electron’s exact location. • This is because of the Heisenberg uncertainty principle. An electron cannot be pinpointed as the photon striking it will cause it to change momentum and position, so you will never be ...
A New Form of Matter (pdf, 217 kB)
... magnetic traps, the atoms overlapped and formed a single giant (by atomic standards) matter wave. Says Ketterle: "Pictures of BECs can be regarded as photographs of wave functions" -- that is, solutions to Schrodinger's equation. Working independently in 1995, Eric Cornell (National Institute of Sta ...
... magnetic traps, the atoms overlapped and formed a single giant (by atomic standards) matter wave. Says Ketterle: "Pictures of BECs can be regarded as photographs of wave functions" -- that is, solutions to Schrodinger's equation. Working independently in 1995, Eric Cornell (National Institute of Sta ...
vsepr_lite_oct_2011 - chemistry11crescentsummer
... fig 5. The Lewis structure of HOH. How many lone pair(s) of electrons are on the central atom, O? Adapt the molecular model of NH3 to get a model of HOH. How would you describe the shape of a water molecule? No fancy name required. Use the bond angles in CH4 and NH3 to predict the H–O–H bond angle i ...
... fig 5. The Lewis structure of HOH. How many lone pair(s) of electrons are on the central atom, O? Adapt the molecular model of NH3 to get a model of HOH. How would you describe the shape of a water molecule? No fancy name required. Use the bond angles in CH4 and NH3 to predict the H–O–H bond angle i ...
Lesson7
... molecule is not restricted to absorbing discrete energies as any additional energy can be taken away by the atoms as kinetic energy which is not quantized. • The shaded area is referred to as a dissociation continuum. ...
... molecule is not restricted to absorbing discrete energies as any additional energy can be taken away by the atoms as kinetic energy which is not quantized. • The shaded area is referred to as a dissociation continuum. ...
AP Chemistry Summer Assignment
... 41.Mercury has an atomic mass of 200.59 amu. Calculate the a.Mass of 3.0 x 1010 atoms. b.Number of atoms in one nanogram of Mercury. 41.Calculate the molar masses ( g/ mol) of a. Ammonia ( NH3) b. Baking soda ( NaHCO3)) c. Osmium Metal (Os) 42.Convert the following to moles a.3.86 grams of Carbon di ...
... 41.Mercury has an atomic mass of 200.59 amu. Calculate the a.Mass of 3.0 x 1010 atoms. b.Number of atoms in one nanogram of Mercury. 41.Calculate the molar masses ( g/ mol) of a. Ammonia ( NH3) b. Baking soda ( NaHCO3)) c. Osmium Metal (Os) 42.Convert the following to moles a.3.86 grams of Carbon di ...
Laser and its applications
... both emission and absorption lines. Let all the atoms emit light of the same wavelength. The effective wavelength observed from those moving towards an observer is diminished and for those atoms moving away it is increased in accordance with Doppler’s principle. When we have a moving source sending ...
... both emission and absorption lines. Let all the atoms emit light of the same wavelength. The effective wavelength observed from those moving towards an observer is diminished and for those atoms moving away it is increased in accordance with Doppler’s principle. When we have a moving source sending ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.