Radiative cascade of highly excited hydrogen atoms in strong magnetic... Türker Topçu and Francis Robicheaux 兲
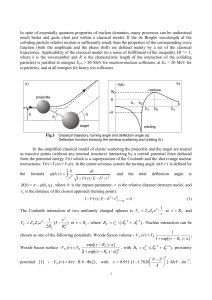
... 兩m兩 from 200 to 40 and observed that the motion in states with 兩m兩 ⬃ 200− 80 are adiabatically separable to the extent where no more than 50% of the energy levels are overtaken by the nonadiabatic couplings. As they decreased 兩m兩 further down to 兩m兩 ⬃ 40 the energy spectrum experienced a transition ...
... 兩m兩 from 200 to 40 and observed that the motion in states with 兩m兩 ⬃ 200− 80 are adiabatically separable to the extent where no more than 50% of the energy levels are overtaken by the nonadiabatic couplings. As they decreased 兩m兩 further down to 兩m兩 ⬃ 40 the energy spectrum experienced a transition ...
Earth`s Tectonic Plates
... and it decays into strontium-87 (the daughter). In order to find the age (T) of a rock or mineral containing atoms of these elements, the following must be known. D is the number of atoms of the daughter product today, while P is the number of atoms of the parent radioisotope today. How are these fi ...
... and it decays into strontium-87 (the daughter). In order to find the age (T) of a rock or mineral containing atoms of these elements, the following must be known. D is the number of atoms of the daughter product today, while P is the number of atoms of the parent radioisotope today. How are these fi ...
Chapter 5. ACIDITY AND BASICITY OF ORGANIC COMPOUNDS
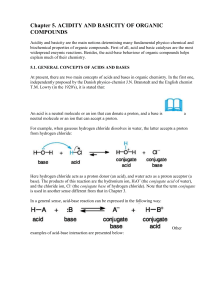
... Another stabilizing factor is the polarizability (opposed to polarity) of an element in the acidic site. This term means the ability of the electrons to respond to a changing electric field, as a result of its interaction with solvent or with other polar reagents. Relative polarizability increases ...
... Another stabilizing factor is the polarizability (opposed to polarity) of an element in the acidic site. This term means the ability of the electrons to respond to a changing electric field, as a result of its interaction with solvent or with other polar reagents. Relative polarizability increases ...
Thesis - Wichita State University
... of an appropriate voltage is given to an IPMC membrane, the charge distribution inside material exerts electric field on its surface. This potential gradient across the surface of IPMC allows flow of positive ions inside the material. When submerged in water, it dissociates molecule of water into it ...
... of an appropriate voltage is given to an IPMC membrane, the charge distribution inside material exerts electric field on its surface. This potential gradient across the surface of IPMC allows flow of positive ions inside the material. When submerged in water, it dissociates molecule of water into it ...
2. Nuclear models and stability
... The aim of this chapter is to understand how certain combinations of N neutrons and Z protons form bound states and to understand the masses, spins and parities of those states. The known (N, Z) combinations are shown in Fig. 2.1. The great majority of nuclear species contain excess neutrons or prot ...
... The aim of this chapter is to understand how certain combinations of N neutrons and Z protons form bound states and to understand the masses, spins and parities of those states. The known (N, Z) combinations are shown in Fig. 2.1. The great majority of nuclear species contain excess neutrons or prot ...
Advanced Higher Chemistry Resource Guide
... quantum number, n, is the circumference of the orbital in terms of the number of wavelengths. Revised Higher 2(a) atomic orbital notes page 28. The RSC website has pages which offer very clear and attractive representations of orbitals with accompanying text which refers to the wave nature of the el ...
... quantum number, n, is the circumference of the orbital in terms of the number of wavelengths. Revised Higher 2(a) atomic orbital notes page 28. The RSC website has pages which offer very clear and attractive representations of orbitals with accompanying text which refers to the wave nature of the el ...
physics - Textbooks Online
... 6.2 Atom models As far back as 1803, Dalton, an English teacher, showed that the matter is made up of extremely small particles called atoms. Prout (1815), suggested that all elements are made up of atoms of hydrogen. Since many of the elements were found to have atomic weights that were not exact m ...
... 6.2 Atom models As far back as 1803, Dalton, an English teacher, showed that the matter is made up of extremely small particles called atoms. Prout (1815), suggested that all elements are made up of atoms of hydrogen. Since many of the elements were found to have atomic weights that were not exact m ...
PHYS 221 General Physics I Course Outcome Summary Course
... changes in some of the ways scientists view the world. By studying the problems that engage today's scientists, students learn to appreciate the importance of science in their lives and to understand the value of a scientific perspective. Students should be encouraged to study both the biological a ...
... changes in some of the ways scientists view the world. By studying the problems that engage today's scientists, students learn to appreciate the importance of science in their lives and to understand the value of a scientific perspective. Students should be encouraged to study both the biological a ...
Slide 1 - MrCard.Org
... 4. While using the clamp to hold the test tube, use the stirring rod to push the steel wool to the bottom of the test tube. Do not pack it, but make sure the wool is at the bottom. 5. Pour enough hydrogen peroxide into the test tube so the steel wool is barely covered. 6. Place the thermometer in th ...
... 4. While using the clamp to hold the test tube, use the stirring rod to push the steel wool to the bottom of the test tube. Do not pack it, but make sure the wool is at the bottom. 5. Pour enough hydrogen peroxide into the test tube so the steel wool is barely covered. 6. Place the thermometer in th ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.