Perspective: Fifty years of density-functional theory in chemical physics
... quantum chemistry community. The accuracy of the HFS model was no match for their well-established wave-function methods. Solid-state physicists, on the other hand, made key advances in KS-DFT in its early decades. Reference 2 surveys the progress and literature of the period. Most importantly, a ri ...
... quantum chemistry community. The accuracy of the HFS model was no match for their well-established wave-function methods. Solid-state physicists, on the other hand, made key advances in KS-DFT in its early decades. Reference 2 surveys the progress and literature of the period. Most importantly, a ri ...
Fundamentals of Semiconductors
... and its alloys. New techniques, such as Raman scattering of x-rays, have given detailed information about the vibrational spectra of the nitrides, available only as thin films or as very small single crystals. An example of the progress in semiconductor physics is our understanding of the class of d ...
... and its alloys. New techniques, such as Raman scattering of x-rays, have given detailed information about the vibrational spectra of the nitrides, available only as thin films or as very small single crystals. An example of the progress in semiconductor physics is our understanding of the class of d ...
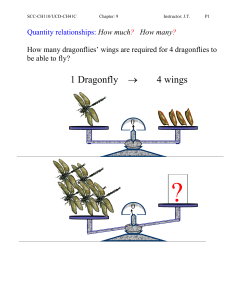
Quantity relationships: How much
... 1.30L of gaseous ethylene is burned. What volume of oxygen is required if both gas volume are measured at STP? → 2CO2 (g) + 2 H2O(l) ...
... 1.30L of gaseous ethylene is burned. What volume of oxygen is required if both gas volume are measured at STP? → 2CO2 (g) + 2 H2O(l) ...
Chemistry Final Exam Review
... ____ 31. Stoichiometry deals with the mass relationships in a chemical reaction. ____ 32. A mole-to-mole ratio converts between two substances in the same chemical reaction. ____ 33. A mole ratio is used to convert between moles and grams. ____ 34. A mole-to-mass calculation converts between the gra ...
... ____ 31. Stoichiometry deals with the mass relationships in a chemical reaction. ____ 32. A mole-to-mole ratio converts between two substances in the same chemical reaction. ____ 33. A mole ratio is used to convert between moles and grams. ____ 34. A mole-to-mass calculation converts between the gra ...
KCl + O KClO 3 → However, this equation is not balanced, since
... All chemical equations must adhere to the natural law of conservation of mass. In terms of the fundamental units of matter (i.e., the atoms of the substances entering the chemical reaction), this law means that each atom placed in the chemical reaction as a reactant must appear in the products of th ...
... All chemical equations must adhere to the natural law of conservation of mass. In terms of the fundamental units of matter (i.e., the atoms of the substances entering the chemical reaction), this law means that each atom placed in the chemical reaction as a reactant must appear in the products of th ...
48th CHEMISTRY OLYMPIAD CHEMISTRY
... 4. Chemical element X acts as a very strong oxidizing agent. Most of the reactions, in which participates, proceed with explosion or with the formation of a flame. At contact with many metals and nonmetals the reaction takes place already at ambient temperature. Even steam and glass wool (combustibl ...
... 4. Chemical element X acts as a very strong oxidizing agent. Most of the reactions, in which participates, proceed with explosion or with the formation of a flame. At contact with many metals and nonmetals the reaction takes place already at ambient temperature. Even steam and glass wool (combustibl ...
No Slide Title
... number. The Standard model does not give any physical meaning for the wave function. Dtheory explains geometrically with help of its space model what are the wave function and its complex phase and how does the gauge principle occur. The developers of the gauge principle considered it to be against ...
... number. The Standard model does not give any physical meaning for the wave function. Dtheory explains geometrically with help of its space model what are the wave function and its complex phase and how does the gauge principle occur. The developers of the gauge principle considered it to be against ...
Quantum Computer (Information) and Quantum Mechanical
... Abstract: Perception may not be what you think it is. Perception is not just a collection of inputs from our sensory system. Instead, it is the brain's interpretation (positive, negative or neutral-no signature case) of stimuli which is based on an individual's genetics and past experiences. Percept ...
... Abstract: Perception may not be what you think it is. Perception is not just a collection of inputs from our sensory system. Instead, it is the brain's interpretation (positive, negative or neutral-no signature case) of stimuli which is based on an individual's genetics and past experiences. Percept ...
College Chemistry
... Atoms and Isotopes In the atomic theory proposed by John Dalton in 1805, all atoms of a given element were assumed to be identical. Eventually it was realized that atoms of a given element are not necessarily identical; an element can exist in several isotopic forms that differ in atomic mass. ...
... Atoms and Isotopes In the atomic theory proposed by John Dalton in 1805, all atoms of a given element were assumed to be identical. Eventually it was realized that atoms of a given element are not necessarily identical; an element can exist in several isotopic forms that differ in atomic mass. ...
Problem Set 7
... atomic weight? (Think Units) The average atomic mass is given in amu, while atomic weight is typically expressed in grams per mole. More typically the term molar mass is used to express grams per mole amounts. 9) Convert the following masses into moles for each element given. a. 3.45 grams of tin x ...
... atomic weight? (Think Units) The average atomic mass is given in amu, while atomic weight is typically expressed in grams per mole. More typically the term molar mass is used to express grams per mole amounts. 9) Convert the following masses into moles for each element given. a. 3.45 grams of tin x ...
Chemistry (Revised)
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
Sample Chapter 3
... beans or rice, but we count eggs or pencils. And we use mass units (a kilogram of coffee beans) or counting units (a dozen pencils) to express the amount. Similarly, daily life in the laboratory involves measuring substances. We want to know the numbers of chemical entities—atoms, ions, molecules, o ...
... beans or rice, but we count eggs or pencils. And we use mass units (a kilogram of coffee beans) or counting units (a dozen pencils) to express the amount. Similarly, daily life in the laboratory involves measuring substances. We want to know the numbers of chemical entities—atoms, ions, molecules, o ...
Unit 2: Matter as Solutions and Gases
... 2. Polar Molecule: - unequal charged distribution due to the electron pairs around the oxygen atom. 3. Strong O−H Hydrogen Bond: - a type of hydrogen bond that is fairly strong compared to other types of intermolecular bonds (bonds between molecules). ...
... 2. Polar Molecule: - unequal charged distribution due to the electron pairs around the oxygen atom. 3. Strong O−H Hydrogen Bond: - a type of hydrogen bond that is fairly strong compared to other types of intermolecular bonds (bonds between molecules). ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... though this fire does not have much in common with a coal-burning power plant. Both the fire and the power plant, however, are technologies that harness energy-producing processes. Humans continually devise new technologies that use chemical reactions to produce materials with useful properties. Sin ...
... though this fire does not have much in common with a coal-burning power plant. Both the fire and the power plant, however, are technologies that harness energy-producing processes. Humans continually devise new technologies that use chemical reactions to produce materials with useful properties. Sin ...
Topic 1 Quantitative Chemistry Answers - slider-dpchemistry-11
... A substance that cannot be divided into simpler, smaller substances. In an element, all the atoms have the same number of protons or electrons, but the number of neutrons may vary (more about this Topic 2) b) atom The smallest part of an element that can exist. An atom consists of an extremely tiny ...
... A substance that cannot be divided into simpler, smaller substances. In an element, all the atoms have the same number of protons or electrons, but the number of neutrons may vary (more about this Topic 2) b) atom The smallest part of an element that can exist. An atom consists of an extremely tiny ...
2013 - SQA
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
Phys114 -2013 Sample Problems ____ 1. A bullet is fired through a
... 44. Jane and Jake are looking at what happens to body 1 of mass m and body 2 of mass 2m, initially at rest, when equal forces are applied separately to the two bodies. Jake says that equal forces applied for equal times do equal amounts of work on the two bodies. Jane says that the two forces do equ ...
... 44. Jane and Jake are looking at what happens to body 1 of mass m and body 2 of mass 2m, initially at rest, when equal forces are applied separately to the two bodies. Jake says that equal forces applied for equal times do equal amounts of work on the two bodies. Jane says that the two forces do equ ...
Science 9 Year End Review The following information includes all
... _________________ will speed up a reaction. Decreasing these will slow it down. Rusting is one type of _________________. Define CORROSION and provide an example (including an equation) Coating a corrosive metal (a metal that can corrode) with zinc is referred to as _________________. Define ...
... _________________ will speed up a reaction. Decreasing these will slow it down. Rusting is one type of _________________. Define CORROSION and provide an example (including an equation) Coating a corrosive metal (a metal that can corrode) with zinc is referred to as _________________. Define ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.