IB Chemistry Review. Unit I. Topics 2
... C. Each magnesium atom loses two electrons and each chlorine atom gains one electron. D. Each magnesium atom gains one electron and each chlorine atom loses two electrons. 6. Which is the best description of ionic bonding? A. The electrostatic attraction between positively charged nuclei and an elec ...
... C. Each magnesium atom loses two electrons and each chlorine atom gains one electron. D. Each magnesium atom gains one electron and each chlorine atom loses two electrons. 6. Which is the best description of ionic bonding? A. The electrostatic attraction between positively charged nuclei and an elec ...
Lesson 1 - Working With Chemicals
... have a nucleus showing the number of protons and neutrons and circles outside the nucleus showing the number of electrons. Reminder: # of protons = # of electrons = atomic # e.g. Draw the Bohr model for the following elements: ...
... have a nucleus showing the number of protons and neutrons and circles outside the nucleus showing the number of electrons. Reminder: # of protons = # of electrons = atomic # e.g. Draw the Bohr model for the following elements: ...
AP Chemistry
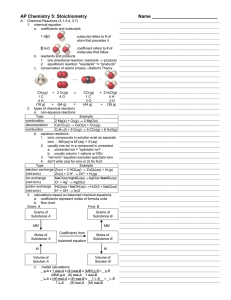
... products of a chemical reaction is the same as the total mass of the reactants. Likewise, the same numbers of atoms of each type are present before and after a chemical reaction. A balanced chemical equation shows equal numbers of atoms of each element on each side of the equation. Equations are bal ...
... products of a chemical reaction is the same as the total mass of the reactants. Likewise, the same numbers of atoms of each type are present before and after a chemical reaction. A balanced chemical equation shows equal numbers of atoms of each element on each side of the equation. Equations are bal ...
Igcse chemistry lesson 2
... reactions studied in this specification 1.19 use the state symbols (s), (l), (g) and (aq) in chemical equations to represent solids, liquids, gases and aqueous solutions respectively 1.20 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water an ...
... reactions studied in this specification 1.19 use the state symbols (s), (l), (g) and (aq) in chemical equations to represent solids, liquids, gases and aqueous solutions respectively 1.20 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water an ...
Thermodynamics - WordPress.com
... 43. The energy possessed by the system due to its nature, chemical composition and thermodynamic state. 44. W = n Cv(T2 – T1) for ‘n’ moles of a gas. 45. The variables like temperature, pressure, volume etc, which define the state of a system are called state functions. 46. Condition of the system e ...
... 43. The energy possessed by the system due to its nature, chemical composition and thermodynamic state. 44. W = n Cv(T2 – T1) for ‘n’ moles of a gas. 45. The variables like temperature, pressure, volume etc, which define the state of a system are called state functions. 46. Condition of the system e ...
Chemical Reactions
... Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: Nitrogen and oxygen react to form nitrogen ...
... Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: Nitrogen and oxygen react to form nitrogen ...
Thermochemistry Note
... This is another method to determine enthalpy for a reaction without having to perform calorimetry and a shortcut to Hess’ law depending on the info available. Molar heats of formation can be found in the data booklet for many substances. The molar heat of formation is defined as the amount of heat r ...
... This is another method to determine enthalpy for a reaction without having to perform calorimetry and a shortcut to Hess’ law depending on the info available. Molar heats of formation can be found in the data booklet for many substances. The molar heat of formation is defined as the amount of heat r ...
mole concept and stoichiometry
... The Law States that , “The ratio of the weights of two elements, A and B which combine separately with a fixed weight of the third element C is either the same or some simple multiple of the ratio of the weights in which A and B combine directly with each other.” He introduced the term “Stoichiometr ...
... The Law States that , “The ratio of the weights of two elements, A and B which combine separately with a fixed weight of the third element C is either the same or some simple multiple of the ratio of the weights in which A and B combine directly with each other.” He introduced the term “Stoichiometr ...
Fugacity model
... Calculated descriptors on the other hand, do not require that the substance is isolated in the laboratory; it may not even have been synthesized, since all that is needed is the chemical structure. A further classification of calculated descriptors is into zero, one, two and three dimensional depend ...
... Calculated descriptors on the other hand, do not require that the substance is isolated in the laboratory; it may not even have been synthesized, since all that is needed is the chemical structure. A further classification of calculated descriptors is into zero, one, two and three dimensional depend ...
Mole - My CCSD
... Is calculated using the atomic mass of each element in the compound, times how many atoms of each element are in the compound. ...
... Is calculated using the atomic mass of each element in the compound, times how many atoms of each element are in the compound. ...
U6B _13-14
... Net Ionic Reactions Shows the details of aqueous reactions that involve ions in aqueous solution Molecular Equation: the typical equation you are use to writing ...
... Net Ionic Reactions Shows the details of aqueous reactions that involve ions in aqueous solution Molecular Equation: the typical equation you are use to writing ...
A Voyage through Equations
... 3. Solid iron (III) oxide and carbon monoxide react to produce iron metal and carbon dioxide gas. Fe2O3 + 3CO 2Fe + 3CO2 4. Sulfuric acid and sodium hydroxide react to form sodium sulfate and water. H2SO4 + 2NaOH Na2SO4 + 2H2O 5. Vanadium (II) oxide with iron (III) Oxide results in the formation ...
... 3. Solid iron (III) oxide and carbon monoxide react to produce iron metal and carbon dioxide gas. Fe2O3 + 3CO 2Fe + 3CO2 4. Sulfuric acid and sodium hydroxide react to form sodium sulfate and water. H2SO4 + 2NaOH Na2SO4 + 2H2O 5. Vanadium (II) oxide with iron (III) Oxide results in the formation ...
CHEMISTRY The Central Science 9th Edition
... by the symbol Co, ……, Nitrogen is represented by the symbol N and Nickel by the symbol Ni, etc…. In general we represent the elements by one capital letter or by two letters, the first is in capital form, while the second letter suppose to be in small form. ...
... by the symbol Co, ……, Nitrogen is represented by the symbol N and Nickel by the symbol Ni, etc…. In general we represent the elements by one capital letter or by two letters, the first is in capital form, while the second letter suppose to be in small form. ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.