Chemistry
... - The volume of a gas changes with changes in temperature and pressure - Because of this a volume of gas is usually measured at standard temperature and pressure (STP) - STP is 0 C and 101.3 kPa or 1 atmosphere - The Earth is surrounded by a blanket of air, which we call the atmosphere. It reaches o ...
... - The volume of a gas changes with changes in temperature and pressure - Because of this a volume of gas is usually measured at standard temperature and pressure (STP) - STP is 0 C and 101.3 kPa or 1 atmosphere - The Earth is surrounded by a blanket of air, which we call the atmosphere. It reaches o ...
Science 10 Chem - Holy Trinity Academy
... H2, O2, N2, F2, Cl2, P4, S8 Compound: when two or more elements are chemically combined together. o They can’t be separated by ordinary physical means o Fixed ratio of elements/never change o e.g., water (H2O) and carbon dioxide (CO2). Lesson 5: The evolution of atomic models Dalton/Solid Sphere ...
... H2, O2, N2, F2, Cl2, P4, S8 Compound: when two or more elements are chemically combined together. o They can’t be separated by ordinary physical means o Fixed ratio of elements/never change o e.g., water (H2O) and carbon dioxide (CO2). Lesson 5: The evolution of atomic models Dalton/Solid Sphere ...
Chemistry: Percent Yield
... 17: 3.1cc A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC sy ...
... 17: 3.1cc A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC sy ...
Final Exam Review whole thing
... A molecule is the smallest unit of an element or compound that retains all of the properties of that element or compound Example tubes of H and O ...
... A molecule is the smallest unit of an element or compound that retains all of the properties of that element or compound Example tubes of H and O ...
10.2The Mole-Mass Relationship
... Converting from moles of a compound to grams Example: I need 3.00 mol NaCl for an experiment. How many grams is that? Step 1: Find the molar mass Molar mass = 22.09g/mol + 35.45g/mol = 57.54 g/mol Step 2: Use the molar mass like a conversion factor. ...
... Converting from moles of a compound to grams Example: I need 3.00 mol NaCl for an experiment. How many grams is that? Step 1: Find the molar mass Molar mass = 22.09g/mol + 35.45g/mol = 57.54 g/mol Step 2: Use the molar mass like a conversion factor. ...
Complete the following equations
... (a) Reaction of calcium metal with water to form hydrogen gas and aqueous calcium hydroxide. Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g); (b) Reaction of solid calcium carbonate with dilute hydrochloric acid and the products are aqueous calcium chloride, water, and carbon dioxide gas. CaCO3(s) + 2HCl(aq) ...
... (a) Reaction of calcium metal with water to form hydrogen gas and aqueous calcium hydroxide. Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g); (b) Reaction of solid calcium carbonate with dilute hydrochloric acid and the products are aqueous calcium chloride, water, and carbon dioxide gas. CaCO3(s) + 2HCl(aq) ...
Equilibrium
... b. If raising the temperature of the reaction results in an equilibrium with a higher concentration of C than A, how will the value of Keq change? 12. The following reaction occurs when steam is passed over hot carbon. The mixture of gases it generates is called water gas and is useful as an indust ...
... b. If raising the temperature of the reaction results in an equilibrium with a higher concentration of C than A, how will the value of Keq change? 12. The following reaction occurs when steam is passed over hot carbon. The mixture of gases it generates is called water gas and is useful as an indust ...
No Slide Title
... The value for the enthalpy change for a process depends not only on the identity and amounts of the reactants and products but also their state. Consider the following example: 2 H2(g) + O2(g) 2 H2O(l) ...
... The value for the enthalpy change for a process depends not only on the identity and amounts of the reactants and products but also their state. Consider the following example: 2 H2(g) + O2(g) 2 H2O(l) ...
110 exam i material
... b. Indicate which of the following is kinetic or potential energy: A rock at the top of a mountain __________________________ an arrow being shot __________________________ the space shuttle lifting off __________________________ c. Indicate which of the following is a chemical or physical change Bu ...
... b. Indicate which of the following is kinetic or potential energy: A rock at the top of a mountain __________________________ an arrow being shot __________________________ the space shuttle lifting off __________________________ c. Indicate which of the following is a chemical or physical change Bu ...
AP Chemistry
... 1. chemical reactions typically involve breaking bonds between reactant atoms and forming new bonds 2. breaking bonds takes energy chemical system gains bond energy; surroundings lose energy (heat, etc.) 3. forming bonds releases energy chemical system loses energy, surroundings gain energy 4. c ...
... 1. chemical reactions typically involve breaking bonds between reactant atoms and forming new bonds 2. breaking bonds takes energy chemical system gains bond energy; surroundings lose energy (heat, etc.) 3. forming bonds releases energy chemical system loses energy, surroundings gain energy 4. c ...
Combined
... After the addition of marble chips to an excess of dilute hydrochloric acid in a conical flask, each of the following was measured and plotted against time on a graph. If the reaction was complete in 2.5 minutes, which of the following, when plotted against time, would give a graph like the one show ...
... After the addition of marble chips to an excess of dilute hydrochloric acid in a conical flask, each of the following was measured and plotted against time on a graph. If the reaction was complete in 2.5 minutes, which of the following, when plotted against time, would give a graph like the one show ...
No Slide Title
... 4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is –1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always –1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to ...
... 4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is –1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always –1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to ...
physical setting chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Determination of the Empirical Formula of an
... small and is probably caused by typical sources of experimental error including, but not limited to, minor weighing errors caused by balance calibration and incomplete reaction of the lead oxide. The experiment would probably have been completed with greater relative ease if the author had used a wi ...
... small and is probably caused by typical sources of experimental error including, but not limited to, minor weighing errors caused by balance calibration and incomplete reaction of the lead oxide. The experiment would probably have been completed with greater relative ease if the author had used a wi ...
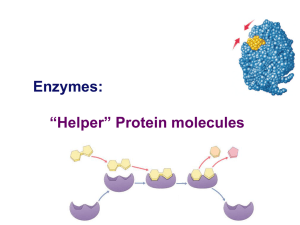
Enzymes: “Helper” Protein molecules
... sucrase breaks down sucrose Oh, I get it! They end in -ase ...
... sucrase breaks down sucrose Oh, I get it! They end in -ase ...
Biodiesel preparation in batch emulgation reactor
... neutralization of catalyst by carbon dioxide (see 3.3.2) and the flash point depends on conditions of demethanolisation, which were constant in all experiments. The yield of EP (Y 1 ) increases with increasing molar ratio of methanol to oil and decreases with decreasing amounts of catalyst and inten ...
... neutralization of catalyst by carbon dioxide (see 3.3.2) and the flash point depends on conditions of demethanolisation, which were constant in all experiments. The yield of EP (Y 1 ) increases with increasing molar ratio of methanol to oil and decreases with decreasing amounts of catalyst and inten ...
Physical Setting/Chemistry Examination
... Answer all questions in this part. Directions (66–83): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 and 67 on the information below. In 1897, J. J. Thoms ...
... Answer all questions in this part. Directions (66–83): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 and 67 on the information below. In 1897, J. J. Thoms ...
Review Outline for Atomic Structure Test
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
... J) Draw the electron dot diagram (Lewis Dot Structure) and then tell if it would give up or take on electrons to get a full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
Naming Compounds
... determine the elements involved in the chemical formula (compound)…. Metals and Non- Metals determine the type of compound (Ionic or Molecular) follow the rules outline for Ionic or Molecular ...
... determine the elements involved in the chemical formula (compound)…. Metals and Non- Metals determine the type of compound (Ionic or Molecular) follow the rules outline for Ionic or Molecular ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.