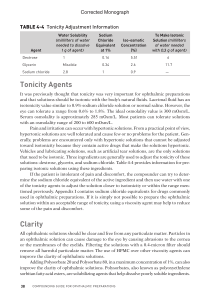
Tonicity Agents Clarity - Pharmacists Provide Care
... It was previously thought that tonicity was very important for ophthalmic preparations and that solutions should be isotonic with the body’s natural fluids. Lacrimal fluid has an isotonicity value similar to 0.9% sodium chloride solution or normal saline. However, the eye can tolerate a range from 0 ...
... It was previously thought that tonicity was very important for ophthalmic preparations and that solutions should be isotonic with the body’s natural fluids. Lacrimal fluid has an isotonicity value similar to 0.9% sodium chloride solution or normal saline. However, the eye can tolerate a range from 0 ...
CASE 4-16180/-JCIP APPENDIX F
... submitted to this IND with respect to the issue of ‘acute renal failure’. The contents of the investigator aieti for this case is included and contains what actions Novartis has taken and will take. ...
... submitted to this IND with respect to the issue of ‘acute renal failure’. The contents of the investigator aieti for this case is included and contains what actions Novartis has taken and will take. ...
Read Document - MotherToBaby
... “The target market is primarily young adults, of whom half are women of reproductive age. The tragic consequences for pregnant users are isotretinoinexposed pregnancies that result in entirely preventable major birth defects and miscarriages. The FDA must step up its oversight. We have recommended t ...
... “The target market is primarily young adults, of whom half are women of reproductive age. The tragic consequences for pregnant users are isotretinoinexposed pregnancies that result in entirely preventable major birth defects and miscarriages. The FDA must step up its oversight. We have recommended t ...
Epidural Steroid Injections Safety Recommendations by the Multi
... Society (ISIS) stated that they felt the alert was misleading in its message regarding the safety of epidural steroid injections and contained inaccuracies regarding the effectiveness of this procedure (19). However, they have approved 17 safety recommendations identical to those considered by the S ...
... Society (ISIS) stated that they felt the alert was misleading in its message regarding the safety of epidural steroid injections and contained inaccuracies regarding the effectiveness of this procedure (19). However, they have approved 17 safety recommendations identical to those considered by the S ...
Safe Handling of Hazardous Drugs
... Hazardous Drug: Defined by the American Society of Health System Pharmacists in 1990 as being a drug which displays one or more of the following characteristics: genotoxicity, carcinogenicity, teratogenicity or fertility impairment, or serious organ or other toxic manifestation at low doses in exper ...
... Hazardous Drug: Defined by the American Society of Health System Pharmacists in 1990 as being a drug which displays one or more of the following characteristics: genotoxicity, carcinogenicity, teratogenicity or fertility impairment, or serious organ or other toxic manifestation at low doses in exper ...
Secundum Artem
... Loyd V. Allen, Jr., Ph.D., R.Ph., Professor Emeritus, University of Oklahoma, HSC College of Pharmacy, Oklahoma City, OK 73190 and Martin A. Erickson III, R.Ph. ...
... Loyd V. Allen, Jr., Ph.D., R.Ph., Professor Emeritus, University of Oklahoma, HSC College of Pharmacy, Oklahoma City, OK 73190 and Martin A. Erickson III, R.Ph. ...
Chapter 8 - Denali Rx
... appropriate quantity and dosage form, from several pharmaceutical ingredients in response to a prescription written by a physician, such as tablets, capsules, ointments, or creams; sometimes referred to as extemporaneous compounding © Paradigm Publishing, Inc. ...
... appropriate quantity and dosage form, from several pharmaceutical ingredients in response to a prescription written by a physician, such as tablets, capsules, ointments, or creams; sometimes referred to as extemporaneous compounding © Paradigm Publishing, Inc. ...
The FDA’s Continuing Incapacity on Livestock Antibiotics *
... explain here, however, the initiatives leave much to be desired, for four basic reasons. First, the success of the FDA’s policy on “judicious use” depends on multiple layers of voluntary action by profit-maximizing drug companies. Although early indications of drug companies’ cooperation are promisi ...
... explain here, however, the initiatives leave much to be desired, for four basic reasons. First, the success of the FDA’s policy on “judicious use” depends on multiple layers of voluntary action by profit-maximizing drug companies. Although early indications of drug companies’ cooperation are promisi ...
Fall Newsletter September 2015
... It shouldn’t come as a surprise that diabetes is a prevalent disease for members of Gold Coast Health Plan (GCHP) and for costs to the Plan. In terms of pharmacy costs, diabetes is the most expensive disease. In the first six months of 2015, GCHP spent $11.1 million on providing medications and supp ...
... It shouldn’t come as a surprise that diabetes is a prevalent disease for members of Gold Coast Health Plan (GCHP) and for costs to the Plan. In terms of pharmacy costs, diabetes is the most expensive disease. In the first six months of 2015, GCHP spent $11.1 million on providing medications and supp ...
Comments Submitted by Immel Resources LLC
... cleaning or adding materials to the batch is problematic – because an error in those operations would be difficult to detect (you can only test for what you know to test for). The error may not be discovered prior to distribution, and may result in patient injury and product recall. We have had nume ...
... cleaning or adding materials to the batch is problematic – because an error in those operations would be difficult to detect (you can only test for what you know to test for). The error may not be discovered prior to distribution, and may result in patient injury and product recall. We have had nume ...
Volume 13.4
... However, in the 1970s, there was a resurgence of pharmacy compounding and in 1985, the USP Convention passed two resolutions urging the USP to become active again in promoting standards for pharmacy compounding. This was followed with convention resolutions in 1990, 1995 and again in 2000. In 1993, ...
... However, in the 1970s, there was a resurgence of pharmacy compounding and in 1985, the USP Convention passed two resolutions urging the USP to become active again in promoting standards for pharmacy compounding. This was followed with convention resolutions in 1990, 1995 and again in 2000. In 1993, ...
Chapter Pharmaceutical Compounding – Nonsterile
... • therapeutic appropriateness and route of administration, including local and systemic biological disposition • legal limitations, if any 2. A Master Formulation Record should be created before compounding a preparation for the first time. This record shall be followed each time that preparation is ...
... • therapeutic appropriateness and route of administration, including local and systemic biological disposition • legal limitations, if any 2. A Master Formulation Record should be created before compounding a preparation for the first time. This record shall be followed each time that preparation is ...
pharmaceutical compounding-nonsterile preparations
... • therapeutic appropriateness and route of administration, including local and systemic biological disposition • legal limitations, if any 2. A Master Formulation Record should be created before compounding a preparation for the first time. This record shall be followed each time that preparation is ...
... • therapeutic appropriateness and route of administration, including local and systemic biological disposition • legal limitations, if any 2. A Master Formulation Record should be created before compounding a preparation for the first time. This record shall be followed each time that preparation is ...
Compounded Topical Anesthetics in Orthodontics
... cream, gel, or ointment base is added to the mixture as a vehicle to carry the drug. PCCA’s plasticized polyethylene-and-mineral-oil gel base is commonly used for topical and oral preparations because it has a soft feel and is completely anhydrous, ideal for water-sensitive active ingredients. Final ...
... cream, gel, or ointment base is added to the mixture as a vehicle to carry the drug. PCCA’s plasticized polyethylene-and-mineral-oil gel base is commonly used for topical and oral preparations because it has a soft feel and is completely anhydrous, ideal for water-sensitive active ingredients. Final ...
The Post-Amarin Age and Its Potential Effect on Off
... and DOJ could find a way to initiate cases outside of the Second Circuit. It could possibly get different results in other U.S. circuits if it found a way to pursue this avenue. As a consequence, the Amarin holding, which is likely to be adopted into the official settlement, should not create an ove ...
... and DOJ could find a way to initiate cases outside of the Second Circuit. It could possibly get different results in other U.S. circuits if it found a way to pursue this avenue. As a consequence, the Amarin holding, which is likely to be adopted into the official settlement, should not create an ove ...
Novartis Pharmaceuticals to Pay $390 Million
... recommending the drug Exjade to Medicaid and Medicare patients. Under the settlement, Novartis has agreed to pay $390 million. The settlement stems from a whistleblower lawsuit, U.S. ex rel. Kester, et al. v. Novartis Pharmaceuticals Corporation, et al., No. 11-CIV-8196, which was filed in the Unite ...
... recommending the drug Exjade to Medicaid and Medicare patients. Under the settlement, Novartis has agreed to pay $390 million. The settlement stems from a whistleblower lawsuit, U.S. ex rel. Kester, et al. v. Novartis Pharmaceuticals Corporation, et al., No. 11-CIV-8196, which was filed in the Unite ...
Maximizing the Use of Single
... • CDC MMWR, Vol. 61, No.27 July 13, 2012. Invasive Staphylococcus aureus Infections Associated with Pain Injections and Reuse of Single-Dose Vials – This report summarizes the investigation of two outbreaks of invasive Staphylococcus aureus infection confirmed in 10 patients being treated for pain i ...
... • CDC MMWR, Vol. 61, No.27 July 13, 2012. Invasive Staphylococcus aureus Infections Associated with Pain Injections and Reuse of Single-Dose Vials – This report summarizes the investigation of two outbreaks of invasive Staphylococcus aureus infection confirmed in 10 patients being treated for pain i ...
schering-plough gmp consent decree puts drug
... vigorously contesting lawsuits claiming to the contrary. “[I]n all adverse event reports involving deaths where an albuterol canister was tested by the company, the canister was shown to contain active ingredient,” according to the firm. “Further, every inhaler involved in a patient’s claim of injur ...
... vigorously contesting lawsuits claiming to the contrary. “[I]n all adverse event reports involving deaths where an albuterol canister was tested by the company, the canister was shown to contain active ingredient,” according to the firm. “Further, every inhaler involved in a patient’s claim of injur ...
navigating the fda
... • Clinical data not supportive of indications for use – need more data •Inadequacy on how people respond to various dosages – need more data •“Approvable” – probably be approved, provided certain issues get resolved •Labeling •Safety issues •Manufacturing issues – can delay or deny application. Prod ...
... • Clinical data not supportive of indications for use – need more data •Inadequacy on how people respond to various dosages – need more data •“Approvable” – probably be approved, provided certain issues get resolved •Labeling •Safety issues •Manufacturing issues – can delay or deny application. Prod ...
FDA Warning Letter to LifeCell Corporation 2011-05-11
... 1. Failure to submit an application to the FDA and obtain approval prior to allowing subjects to participate in the investigation. [21 CFR 812.20(a)(1) and (a)(2), 21 CFR 812.40, 21 CFR 812.42] A sponsor is required to submit an IDE application to FDA and obtain FDA approval of the application prior ...
... 1. Failure to submit an application to the FDA and obtain approval prior to allowing subjects to participate in the investigation. [21 CFR 812.20(a)(1) and (a)(2), 21 CFR 812.40, 21 CFR 812.42] A sponsor is required to submit an IDE application to FDA and obtain FDA approval of the application prior ...
Complaint Filed in US District Court, Midland
... This compliance policy guidance is intended to provide guidance and instructions to FDA staff, industry, and the public for obtaining information to help fulfill the Agency’s plans regarding the compounding of drugs for use in animals. The compliance policy guidance does not create or confer any ri ...
... This compliance policy guidance is intended to provide guidance and instructions to FDA staff, industry, and the public for obtaining information to help fulfill the Agency’s plans regarding the compounding of drugs for use in animals. The compliance policy guidance does not create or confer any ri ...
Slides - Food and Drug Law Institute
... and REDUCE-IT trials, several cardiovascular outcomes studies had called into question whether a reduction in triglyceride levels would translate into a reduction in cardiovascular events. • Accordingly, FDA asked the Advisory Committee whether Vascepa®’s triglyceride lowering effect was sufficient ...
... and REDUCE-IT trials, several cardiovascular outcomes studies had called into question whether a reduction in triglyceride levels would translate into a reduction in cardiovascular events. • Accordingly, FDA asked the Advisory Committee whether Vascepa®’s triglyceride lowering effect was sufficient ...
Pharmacy Newsletter - Gold Coast Health Plan
... The Pharmacy Newsletter is published quarterly for the provider community of Gold Coast Health Plan by the Communications Department. Information in the Pharmacy Newsletter comes from a wide range of medical experts. If you have any questions regarding its content, please contact GCHP’s Pharmacy Dir ...
... The Pharmacy Newsletter is published quarterly for the provider community of Gold Coast Health Plan by the Communications Department. Information in the Pharmacy Newsletter comes from a wide range of medical experts. If you have any questions regarding its content, please contact GCHP’s Pharmacy Dir ...
MedWatch - Boca Medical Products, Inc
... FDA Advises Manufacturers to Test Glycerin for Possible Contamination The Food and Drug Administration has issued an advisory statement to manufacturers that they test their glycerin for possible contamination. This advisory follows reports of deaths in other countries attributed to Chinese drug cou ...
... FDA Advises Manufacturers to Test Glycerin for Possible Contamination The Food and Drug Administration has issued an advisory statement to manufacturers that they test their glycerin for possible contamination. This advisory follows reports of deaths in other countries attributed to Chinese drug cou ...
New England Compounding Center meningitis outbreak

A New England Compounding Center meningitis outbreak which began in September 2012 sickened over 800 individuals and resulted in the death of 64. In September 2012, the Centers for Disease Control and Prevention, in collaboration with state and local health departments and the Food and Drug Administration (FDA), began investigating a multistate outbreak of fungal meningitis and other infections among patients who had received contaminated steroid injections from the New England Compounding Center (NECC) in Framingham, Massachusetts. The NECC was classified as a compounding pharmacy. Such pharmacies are authorized to combine, mix, or alter ingredients to create specific formulations of drugs to meet the specific needs of individual patients, and only in response to individual prescriptions.In October 2012, an investigation of the NECC revealed the company had been in violation of its state license because it had been functioning as a drug manufacturer, producing drugs for broad use rather than filling individual prescriptions. In December, federal prosecutors charged 14 former NECC employees, including president Barry Cadden and pharmacist Glenn Chin, with a host of criminal offenses. It alleged that from 2006 to 2012, NECC knowingly sent out drugs that were mislabeled and unsanitary or contaminated.In a congressional hearing the FDA Commissioner was asked why regulators at the FDA and the Massachusetts Board of Pharmacy did not take action against the pharmacy years earlier. The legislators were told that the agency was obligated to defer to Massachusetts authorities, who had more direct oversight over pharmacies. The FDA Commissioner also stated, ""In light of growing evidence of threats to the public health, the administration urges Congress to strengthen standards for non-traditional compounding."" The Drug Quality and Security Act (H.R. 3204), a bill to grant the FDA more authority to regulate and monitor the manufacturing of compounding drugs, was passed by the Senate on November 27, 2013.The incident resulted in numerous lawsuits against NECC. In May 2015, a $200 million settlement plan was approved that set aside funds for victims of the outbreak and their families.























