Adding Enzymes To Dairy Diets
... made up of two types of chains: branched amylopectin and linear amylose. Xylanases are involved in the digestion of hemicellulose, which has a xylan backbone. As figure 1 suggests, each enzyme has a unique three-dimensional structure which is critical to its ability to bind specific substrate. If th ...
... made up of two types of chains: branched amylopectin and linear amylose. Xylanases are involved in the digestion of hemicellulose, which has a xylan backbone. As figure 1 suggests, each enzyme has a unique three-dimensional structure which is critical to its ability to bind specific substrate. If th ...
Stabilization of carbanions
... so that a significant fraction of the alcohol is in the ionized (alkoxide)! form at physiological pH.! ...
... so that a significant fraction of the alcohol is in the ionized (alkoxide)! form at physiological pH.! ...
FES 100 - Introduction to Forest Biology Exam 1: 100 points October
... If a water solution has a concentration of 10-10 mole of OH- ions, how many H+ ions does it ...
... If a water solution has a concentration of 10-10 mole of OH- ions, how many H+ ions does it ...
(pt=2) What is an acid?
... If a water solution has a concentration of 10-10 mole of OH- ions, how many H+ ions does it ...
... If a water solution has a concentration of 10-10 mole of OH- ions, how many H+ ions does it ...
20.3 Factors Affecting Enzyme Activity
... range from 50°C to 120°C. • have enzymes with tertiary structures that are not destroyed by such high temperatures. ...
... range from 50°C to 120°C. • have enzymes with tertiary structures that are not destroyed by such high temperatures. ...
Document
... starch. Amylase is present in our saliva and in secretions from the pancreas, and begins to act on the starch in our food while still in the mouth. Starch is a polymer made from many glucose molecules connected together (figure at right). It is used by many organisms as a way to store glucose for la ...
... starch. Amylase is present in our saliva and in secretions from the pancreas, and begins to act on the starch in our food while still in the mouth. Starch is a polymer made from many glucose molecules connected together (figure at right). It is used by many organisms as a way to store glucose for la ...
Module 13 Enzymes and Vitamins Lecture 34 Enzymes
... The inhibitors can be reversible or nonreversible. In case of reversible inhibitor, the inhibitor binds with the enzyme through intermolecular interactions such as H-bond, ionic bonding and van der Waals interactions. There will be equilibrium between the enzyme-inhibitor complex and free enzyme and ...
... The inhibitors can be reversible or nonreversible. In case of reversible inhibitor, the inhibitor binds with the enzyme through intermolecular interactions such as H-bond, ionic bonding and van der Waals interactions. There will be equilibrium between the enzyme-inhibitor complex and free enzyme and ...
Enzymes - Philadelphia University Jordan
... 2. troponin I are regulatory proteins for myocardial contractility. They are released into the plasma in response to cardiac damage. 3. Cardiac troponin I (cTnI) appears in plasma within 4–6 hours after an MI, peaks in 8–28 hours, and remains elevated for 3–10 days. - Elevated cTnI, in compination w ...
... 2. troponin I are regulatory proteins for myocardial contractility. They are released into the plasma in response to cardiac damage. 3. Cardiac troponin I (cTnI) appears in plasma within 4–6 hours after an MI, peaks in 8–28 hours, and remains elevated for 3–10 days. - Elevated cTnI, in compination w ...
Biology-1 Exam Two Sample Questions Substrates bind to an
... 10. The mitochondrion ATP synthase a. is a nucleic acid complex. b. transports H+ ions from the matrix to the intermembrane space. c. couples the flow of H+ to the phosphorylation of NAD+. d. is embedded in the outer membrane of the mitochondron. e. helps transport H+ against the concentration gradi ...
... 10. The mitochondrion ATP synthase a. is a nucleic acid complex. b. transports H+ ions from the matrix to the intermembrane space. c. couples the flow of H+ to the phosphorylation of NAD+. d. is embedded in the outer membrane of the mitochondron. e. helps transport H+ against the concentration gradi ...
Enzymes: “Helper” Protein molecules
... Each enzyme is the specific helper to a specific reaction each enzyme needs to be the right shape for the job enzymes are named for the reaction they help ...
... Each enzyme is the specific helper to a specific reaction each enzyme needs to be the right shape for the job enzymes are named for the reaction they help ...
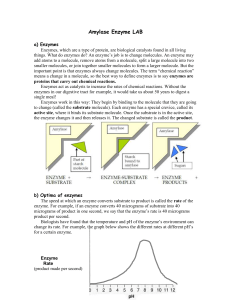
K m + [S]
... ubiqitin-dependent pathways and by ATPindependent pathways. It also Related to the nutrition and hormone state. ...
... ubiqitin-dependent pathways and by ATPindependent pathways. It also Related to the nutrition and hormone state. ...
Biological Catalysts
... The diagram above shows the different levels a protein molecule is made up of, finishing off with a globular structure. First comes the primary structure made up of a sequence of amino acids joined together, followed by the secondary structure where the amino acid chains are bonded by Hydrogen bond ...
... The diagram above shows the different levels a protein molecule is made up of, finishing off with a globular structure. First comes the primary structure made up of a sequence of amino acids joined together, followed by the secondary structure where the amino acid chains are bonded by Hydrogen bond ...
INDUCTION OF ß-GALACTOSIDASE IN E.COLI
... the binding of polymerase and transcription. Lactose, isopropylthiogalactoside and other inducers can bind to the repressor protein removing it from the operator, so RNA polymerase can move through the operator to transcribe the lac operon. The mRNAs are translated and the elevated protein productio ...
... the binding of polymerase and transcription. Lactose, isopropylthiogalactoside and other inducers can bind to the repressor protein removing it from the operator, so RNA polymerase can move through the operator to transcribe the lac operon. The mRNAs are translated and the elevated protein productio ...
StudyGuide_Biochemistry
... 43. What conditions can affect the rate at which a chemical reaction can occur? 44. Enzymes are a type of catalyst. What does that mean? 45. Is an enzyme consumed in a chemical reaction? Explain. 46. How does having enzymes involved in our body’s chemical reactions help us? 47. What is a substrate? ...
... 43. What conditions can affect the rate at which a chemical reaction can occur? 44. Enzymes are a type of catalyst. What does that mean? 45. Is an enzyme consumed in a chemical reaction? Explain. 46. How does having enzymes involved in our body’s chemical reactions help us? 47. What is a substrate? ...
" Enzymes "
... complex. The addition of more substrate will overcome the inhibition, because it will drive the various equilibrium in the direction of the enzyme-substrate complex. The extent of the inhibition will, therefore, depend on: a- Concentration of the inhibitor. b- Concentration of the substrate. c- Rela ...
... complex. The addition of more substrate will overcome the inhibition, because it will drive the various equilibrium in the direction of the enzyme-substrate complex. The extent of the inhibition will, therefore, depend on: a- Concentration of the inhibitor. b- Concentration of the substrate. c- Rela ...
2-7 Active-Site Geometry
... orientation and the collision will be non-productive. Thus, if both molecules first bind to an enzyme active site, and do so in such a way that their reactive portions are juxtaposed, the probability of a reaction is optimized. In solution, when two molecules collide but do not react they bounce off ...
... orientation and the collision will be non-productive. Thus, if both molecules first bind to an enzyme active site, and do so in such a way that their reactive portions are juxtaposed, the probability of a reaction is optimized. In solution, when two molecules collide but do not react they bounce off ...
Structural Biochemistry/Enzyme/Active Site
... is only a small part of the total enzyme volume. It enhances the enzyme to bind to substrate and catalysis by many differnet weak interactions because of its nonpolar microenvironment. The weak interactions includes the Van der Waals, hydrogen bonding, and electrostatic interactions. The arrangement ...
... is only a small part of the total enzyme volume. It enhances the enzyme to bind to substrate and catalysis by many differnet weak interactions because of its nonpolar microenvironment. The weak interactions includes the Van der Waals, hydrogen bonding, and electrostatic interactions. The arrangement ...
Enzymes: “Helper” Protein molecules
... Each enzyme is the specific helper to a specific reaction each enzyme needs to be the right shape for the job enzymes are named for the reaction they help ...
... Each enzyme is the specific helper to a specific reaction each enzyme needs to be the right shape for the job enzymes are named for the reaction they help ...
Outline05 Enzymes - Napa Valley College
... - regulation by non-covalent binding of a modulator to a regulatory site on the enzyme - can be either allosteric activation or allosteric inhibition - reaction rate depends on concentration of the modulator as well as the substrate ...
... - regulation by non-covalent binding of a modulator to a regulatory site on the enzyme - can be either allosteric activation or allosteric inhibition - reaction rate depends on concentration of the modulator as well as the substrate ...
Bio102 Problems
... Both are ultimately expelled from the body as CO2. To get there, each carbon atom from a lipid must be more oxidized more times than each carbon atom from a carbohydrate. With each of these oxidations, a reduced coenzyme is produced which will ultimately be used to synthesize more ATP by oxidative p ...
... Both are ultimately expelled from the body as CO2. To get there, each carbon atom from a lipid must be more oxidized more times than each carbon atom from a carbohydrate. With each of these oxidations, a reduced coenzyme is produced which will ultimately be used to synthesize more ATP by oxidative p ...
Metabolic Pathways
... absence of particular enzymes in the metabolic pathway and through the regulation of the rate of reaction of key enzymes within the pathway. • Regulation can be controlled by intra- and extracellular signal molecules. • Induced fit and the role of the active site of enzymes including shape and subst ...
... absence of particular enzymes in the metabolic pathway and through the regulation of the rate of reaction of key enzymes within the pathway. • Regulation can be controlled by intra- and extracellular signal molecules. • Induced fit and the role of the active site of enzymes including shape and subst ...
Enzyme inhibitor

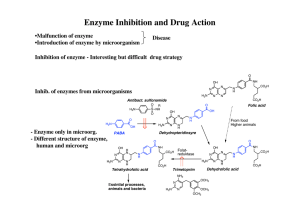
An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.








![K m + [S]](http://s1.studyres.com/store/data/008289247_1-97eed219b6e242b1a447e591c5c01f05-300x300.png)