3.2 Text
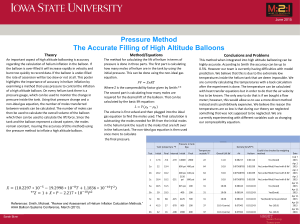
... was known for his inventions, including the hydrogen balloon. Today, Charles is best known for his investigations of the behavior of gases. Charles collected data on the relationship between the temperature and volume of gases. When he graphed the data, the graph was a straight line, as shown in Fig ...
... was known for his inventions, including the hydrogen balloon. Today, Charles is best known for his investigations of the behavior of gases. Charles collected data on the relationship between the temperature and volume of gases. When he graphed the data, the graph was a straight line, as shown in Fig ...
Here
... balance is between Pair and P (pressure by the extra liquid on the right). If Pair changes, the height of the right column, h, will change. You now have a barometer, an instrument that can measure and monitor the atmospheric pressure. Barometers like this are usually filled with mercury. Mercury is ...
... balance is between Pair and P (pressure by the extra liquid on the right). If Pair changes, the height of the right column, h, will change. You now have a barometer, an instrument that can measure and monitor the atmospheric pressure. Barometers like this are usually filled with mercury. Mercury is ...
GBFM-Issue 1-Amendment 0
... valve to open. Should this occur then the tricing line should be used to pull the servo diaphragm up into the balloon allowing the water to escape from the drain valves fitted in the upper part of the servo diaphragm. 3.4.2 Gas valve fails closed If the gas valve fails closed when the balloon is at ...
... valve to open. Should this occur then the tricing line should be used to pull the servo diaphragm up into the balloon allowing the water to escape from the drain valves fitted in the upper part of the servo diaphragm. 3.4.2 Gas valve fails closed If the gas valve fails closed when the balloon is at ...
EFFICIENCY EVALUATIONS OF A BALLOON POWERED CAR
... In our case, the balloon is attached to a cylindrical nozzle and deflates through it (Figure 1). In this case three forces are exerted to the car; the motivational force which is exerted to the balloon due to the momentum of the outgoing air flow, and two resistive forces which are: Aerodynamic dra ...
... In our case, the balloon is attached to a cylindrical nozzle and deflates through it (Figure 1). In this case three forces are exerted to the car; the motivational force which is exerted to the balloon due to the momentum of the outgoing air flow, and two resistive forces which are: Aerodynamic dra ...
Here
... We will first learn about the ideal gas law. That is an equation that tells you which properties of the air inside a balloon work to determine the air's pressure. Then we will look at Charles' Law, a special situation involving the ideal gas law (air temperature volume, and density change together ...
... We will first learn about the ideal gas law. That is an equation that tells you which properties of the air inside a balloon work to determine the air's pressure. Then we will look at Charles' Law, a special situation involving the ideal gas law (air temperature volume, and density change together ...
EFFICIENCY EVALUATIONS OF A BALLOON
... on, is the installation of a filled balloon on the car, with its end pointing towards the back of the car, deflating and causing propulsion (rocket type car). In this case, because of the momentum of the outgoing air jet, a force will be exerted to the car, forcing it to move. There are other design ...
... on, is the installation of a filled balloon on the car, with its end pointing towards the back of the car, deflating and causing propulsion (rocket type car). In this case, because of the momentum of the outgoing air jet, a force will be exerted to the car, forcing it to move. There are other design ...
Here`s
... First is gravity, it pulls downward. The strength of the gravity force (the weight of the air in the parcel) depends on the mass of the air inside the parcel. Second there is an upward pointing pressure difference force. This force is caused by the air outside (surrounding) the parcel. Pressure decr ...
... First is gravity, it pulls downward. The strength of the gravity force (the weight of the air in the parcel) depends on the mass of the air inside the parcel. Second there is an upward pointing pressure difference force. This force is caused by the air outside (surrounding) the parcel. Pressure decr ...
AMEE 202 Midterm S14_1 Group 2
... a. Find the velocity of the surface of the inner cylinder b. Find the Shear Stress on the inner cylinder. c. Find the total force acting on the inner cylinder, and the Torque. ...
... a. Find the velocity of the surface of the inner cylinder b. Find the Shear Stress on the inner cylinder. c. Find the total force acting on the inner cylinder, and the Torque. ...
here
... it is possible to keep pressure constant by changing N and V together in just the right kind of way. This is what happens in Experiment #1 that some of you are working on. Oxygen in a graduated cylinder reacts with steel wool to form rust. Oxygen is removed from the air sample which is a decrease in ...
... it is possible to keep pressure constant by changing N and V together in just the right kind of way. This is what happens in Experiment #1 that some of you are working on. Oxygen in a graduated cylinder reacts with steel wool to form rust. Oxygen is removed from the air sample which is a decrease in ...
Lab 7: Fluids - Physics Department, Princeton University
... or a mathematically described small portion of it, can move, and can also change shape. But each portion has mass and inertia, and can change its momentum only in response to forces. Understanding how the forces that arise between various portions of a fluid sample affect their shapes and motion lea ...
... or a mathematically described small portion of it, can move, and can also change shape. But each portion has mass and inertia, and can change its momentum only in response to forces. Understanding how the forces that arise between various portions of a fluid sample affect their shapes and motion lea ...
Fluid Mechanics Sample Exam 1 Please work at least three
... velocity distribution at the entrance to the tunnel test section is horizontal and uniform, i.e., flat, with a magnitude Uo . Assume that the test section entrance pressure is likewise spatially uniform and equal to Po . Using a pitot tube (or any device/devices that allows simultaneous measurement ...
... velocity distribution at the entrance to the tunnel test section is horizontal and uniform, i.e., flat, with a magnitude Uo . Assume that the test section entrance pressure is likewise spatially uniform and equal to Po . Using a pitot tube (or any device/devices that allows simultaneous measurement ...
Using Tank Pressure To Determine the Lift of a Balloon
... prediction. We believe that this is due to the extremely low temperatures inside the helium tank that we deem impossible. We are currently calculating the temperatures with a back calculation after the experiment is done. The temperature can be calculated with heat transfer equations but in order to ...
... prediction. We believe that this is due to the extremely low temperatures inside the helium tank that we deem impossible. We are currently calculating the temperatures with a back calculation after the experiment is done. The temperature can be calculated with heat transfer equations but in order to ...
DragForce
... where is the density of the medium and CD is a coefficient which depends on the form of the object and the medium. This equation comes from the fact that a falling object sweeps out a volume V = Avt where A is the cross sectional area, v is the velocity and t is the time interval. The number of ...
... where is the density of the medium and CD is a coefficient which depends on the form of the object and the medium. This equation comes from the fact that a falling object sweeps out a volume V = Avt where A is the cross sectional area, v is the velocity and t is the time interval. The number of ...
feb01
... starts to warm up it expands. Density in the balloon decreases. The volume and temperature keep changing in a way that kept pressure constant (pressure inside the balloon is staying equal to the air pressure outside the balloon). Eventually the balloon ends up back at room temperature (unless it pop ...
... starts to warm up it expands. Density in the balloon decreases. The volume and temperature keep changing in a way that kept pressure constant (pressure inside the balloon is staying equal to the air pressure outside the balloon). Eventually the balloon ends up back at room temperature (unless it pop ...
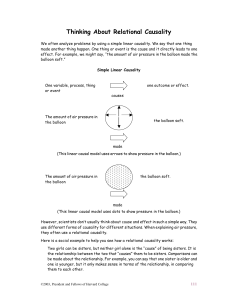
Thinking About Relational Causality
... (This linear causal model uses dots to show pressure in the balloon.) However, scientists don’t usually think about cause and effect in such a simple way. They use different forms of causality for different situations. When explaining air pressure, they often use a relational causality. Here is a so ...
... (This linear causal model uses dots to show pressure in the balloon.) However, scientists don’t usually think about cause and effect in such a simple way. They use different forms of causality for different situations. When explaining air pressure, they often use a relational causality. Here is a so ...
Balloon

A balloon is a flexible bag that can be inflated with a gas, such as helium, hydrogen, nitrous oxide, oxygen, or air. Modern day balloons are made from materials such as rubber, latex, polychloroprene, or a nylon fabric, and can come in many colors. Some early balloons were made of dried animal bladders, such as the pig bladder. Some balloons are used for decorative purposes, while others are used for practical purposes such as meteorology, medical treatment, military defense, or transportation. A balloon's properties, including its low density and low cost, have led to a wide range of applications.The rubber balloon was invented by Michael Faraday in 1824, during experiments with various gases.