Beta decay as a virtual particle interaction analogous to
... proton, the electron is free and clear of the proton, and unlikely to be reabsorbed. Note that the virtual electron-positron pair wavelength at the pair production energy, 1.022 MeV, is 6.07 x 10-13 meters, nearly 700 times the proton radius. It is not necessary for the virtual particle pair to be a ...
... proton, the electron is free and clear of the proton, and unlikely to be reabsorbed. Note that the virtual electron-positron pair wavelength at the pair production energy, 1.022 MeV, is 6.07 x 10-13 meters, nearly 700 times the proton radius. It is not necessary for the virtual particle pair to be a ...
Electron Diffraction Tube
... analyse the distance between the lattice planes of graphite that is d1=213 pm and d2=123 pm. To analyse the inner structure of matter, the bullet particles have to be as small as possible compared to the analysed structure. Light with a wavelength of λ=500 nm e.g. is not appropriate to analyse the p ...
... analyse the distance between the lattice planes of graphite that is d1=213 pm and d2=123 pm. To analyse the inner structure of matter, the bullet particles have to be as small as possible compared to the analysed structure. Light with a wavelength of λ=500 nm e.g. is not appropriate to analyse the p ...
doc
... analyse the distance between the lattice planes of graphite that is d 1=213 pm and d2=123 pm. To analyse the inner structure of matter, the bullet particles have to be as small as possible compared to the analysed structure. Light with a wavelength of λ=500 nm e.g. is not appropriate to analyse the ...
... analyse the distance between the lattice planes of graphite that is d 1=213 pm and d2=123 pm. To analyse the inner structure of matter, the bullet particles have to be as small as possible compared to the analysed structure. Light with a wavelength of λ=500 nm e.g. is not appropriate to analyse the ...
p Well - Purdue Physics
... boundary with total reflection for electrons in the epitaxial silicon because pWell are more heavily doped and electric field in the depletion region will reflect the electron away. ...
... boundary with total reflection for electrons in the epitaxial silicon because pWell are more heavily doped and electric field in the depletion region will reflect the electron away. ...
BALL LIGHTNING AND PLASMA COHESION John J. Gilman
... value of n2 is n2 /3, so the average rR is about 3 cm. At this radius the binding energy of an electron to its positive ion is very small, but the polarizabilty, " . 4BrR3/3 of the average Rydberg atom is very large. It is about 40B cm3 which is about 1025 times larger than that of an individual nit ...
... value of n2 is n2 /3, so the average rR is about 3 cm. At this radius the binding energy of an electron to its positive ion is very small, but the polarizabilty, " . 4BrR3/3 of the average Rydberg atom is very large. It is about 40B cm3 which is about 1025 times larger than that of an individual nit ...
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
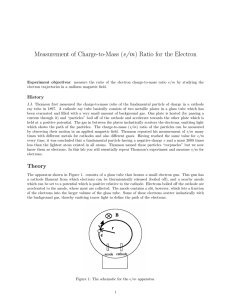
... which shows the path of the particles. The charge-to-mass (e/m) ratio of the particles can be measured by observing their motion in an applied magnetic field. Thomson repeated his measurement of e/m many times with different metals for cathodes and also different gases. Having reached the same value ...
... which shows the path of the particles. The charge-to-mass (e/m) ratio of the particles can be measured by observing their motion in an applied magnetic field. Thomson repeated his measurement of e/m many times with different metals for cathodes and also different gases. Having reached the same value ...
Chapter 4: Old Models of the Atom
... Thomson put this together to come up with a new model of the atom. Basically, he figured out using his own experiments8 that the particles were really really small, and also deduced that they had to come from atoms. To explain this, he came up with the plum pudding model of the atom, which theorizes ...
... Thomson put this together to come up with a new model of the atom. Basically, he figured out using his own experiments8 that the particles were really really small, and also deduced that they had to come from atoms. To explain this, he came up with the plum pudding model of the atom, which theorizes ...