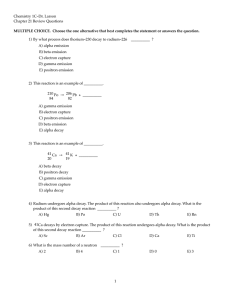
Unit Two Objectives
... b. Democritus: The first to say that matter is composed of atom, or “atomos.” c. Dalton: Had five basic principles in his model of the atom d. Thomson: discovered the charge of the electron by deflecting the flow of electrons through his Cathode Ray Tube with magnetic and electrical fields, and theo ...
... b. Democritus: The first to say that matter is composed of atom, or “atomos.” c. Dalton: Had five basic principles in his model of the atom d. Thomson: discovered the charge of the electron by deflecting the flow of electrons through his Cathode Ray Tube with magnetic and electrical fields, and theo ...
Class Notes 9/23/14 - Physics Internal Website
... Neutral objects get attracted by charged objects as a result of polarization force (due to separation of charge). ...
... Neutral objects get attracted by charged objects as a result of polarization force (due to separation of charge). ...
Electrostatics PP complete
... ◦ Polarization (not really charging, as a polarized object’s NET charge can be zero ) A charged object is brought near a neutral conductor, electrons on the conductor move (toward, or away from the charged object) and then the polarized object behaves as if it has the ________ charge as the object ...
... ◦ Polarization (not really charging, as a polarized object’s NET charge can be zero ) A charged object is brought near a neutral conductor, electrons on the conductor move (toward, or away from the charged object) and then the polarized object behaves as if it has the ________ charge as the object ...
2 The interaction of energetic particles with material
... The interaction of photons with material The three main interactions are: - photo-effect: the photon interacts with a electron which is in a bounded shell. The electron is emitted with an energy equal to the photon energy minus the bounding energy of the electron; - Compton effect: the photon inter ...
... The interaction of photons with material The three main interactions are: - photo-effect: the photon interacts with a electron which is in a bounded shell. The electron is emitted with an energy equal to the photon energy minus the bounding energy of the electron; - Compton effect: the photon inter ...
Vocabulary Lists
... 67. *Simple Harmonic Motion – motion that takes place when the acceleration of an object is proportional to its displacement from its equilibrium position and is always directed toward its equilibrium position (NOTE: this motion is defined by the equation a = -ω2x) 68. Damping – involves a force tha ...
... 67. *Simple Harmonic Motion – motion that takes place when the acceleration of an object is proportional to its displacement from its equilibrium position and is always directed toward its equilibrium position (NOTE: this motion is defined by the equation a = -ω2x) 68. Damping – involves a force tha ...
Glossary File
... In the Standard Model the fundamental interactions are the strong, electromagnetic, weak, and gravitational interactions. Four interaction types are all that are needed to explain all observed physical phenomena. The theory proposes at least one more fundamental interaction that is responsible for f ...
... In the Standard Model the fundamental interactions are the strong, electromagnetic, weak, and gravitational interactions. Four interaction types are all that are needed to explain all observed physical phenomena. The theory proposes at least one more fundamental interaction that is responsible for f ...
K0schoolscenario - Elementary Particle Physics Group
... Quarks make up the Hadrons • Baryons - made up of 3 quarks eg protons & neutrons ...
... Quarks make up the Hadrons • Baryons - made up of 3 quarks eg protons & neutrons ...
File
... electrons through chemical reactions. – This gives them an outer shell configuration like their nearest noble gas and therefore they become stable. – From the family number of the representative elements, you can determine the number of valence electrons, and therefore the number of electrons necess ...
... electrons through chemical reactions. – This gives them an outer shell configuration like their nearest noble gas and therefore they become stable. – From the family number of the representative elements, you can determine the number of valence electrons, and therefore the number of electrons necess ...
CHEM_S1CourseReview_2011
... What property of elements is used to organize the periodic table? How many groups and periods are on the periodic table? What are the family names for groups 1,2,3-12, 17,18? Where are the metals, nonmetals, and metalloids located? How can periodic trends be explained? ...
... What property of elements is used to organize the periodic table? How many groups and periods are on the periodic table? What are the family names for groups 1,2,3-12, 17,18? Where are the metals, nonmetals, and metalloids located? How can periodic trends be explained? ...
E. Rutherford, Proc. Roy. Soc., A97, 374 Bakerian Lecture
... close collisions of swift α-particles with light atoms of matter with the view of determining whether the nuclear structure of some of the lighter atoms could be disintegrated by the intense forces brought into play in such close collisions. Evidence was given that the passage of α- particles throug ...
... close collisions of swift α-particles with light atoms of matter with the view of determining whether the nuclear structure of some of the lighter atoms could be disintegrated by the intense forces brought into play in such close collisions. Evidence was given that the passage of α- particles throug ...
PHY 184 lecture 2
... For example,when a plastic rod is charged by rubbing it with a fur, charge is neither created nor destroyed, but instead electrons are transferred to the rod leaving a net positive charge on the fur. Law of charge conservation • The total charge of an isolated system is strictly conserved. ...
... For example,when a plastic rod is charged by rubbing it with a fur, charge is neither created nor destroyed, but instead electrons are transferred to the rod leaving a net positive charge on the fur. Law of charge conservation • The total charge of an isolated system is strictly conserved. ...
Chemistry2 Midterm Review 2012 – Tuesday
... 61. The equation that is used to calculate the energy of a photon is: 62. Are the following variables inversely proportional or directly proportional: a) frequency and wavelength b) energy and wavelength c) energy and frequency 63. An AM radio station broadcasts at 1440 kHz, and its FM partner broad ...
... 61. The equation that is used to calculate the energy of a photon is: 62. Are the following variables inversely proportional or directly proportional: a) frequency and wavelength b) energy and wavelength c) energy and frequency 63. An AM radio station broadcasts at 1440 kHz, and its FM partner broad ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.