Nuclear Reactions - Kelso High School
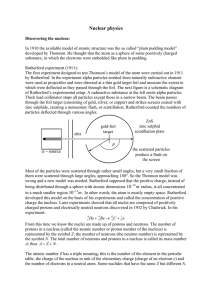
... At the turn of the century, matter was thought to be made from small indivisible particles called atoms. There was, however, evidence to suggest that there were even smaller particles, and an early model of the atom was the ‘plum pudding’ model. This model had to be revised in order to explain the r ...
... At the turn of the century, matter was thought to be made from small indivisible particles called atoms. There was, however, evidence to suggest that there were even smaller particles, and an early model of the atom was the ‘plum pudding’ model. This model had to be revised in order to explain the r ...
Atomic Structure
... Atomic Structure • Rutherford’s Experiment – Alpha particles projected toward gold foil – Expectation: majority will pass through with slight deflection – Results: most have no deflection and a few bounce back entirely ...
... Atomic Structure • Rutherford’s Experiment – Alpha particles projected toward gold foil – Expectation: majority will pass through with slight deflection – Results: most have no deflection and a few bounce back entirely ...
Nuclear physics α −
... Most of the particles were scattered through rather small angles, but a very small fraction of them were scattered through large angles, approaching 180°. So the Thomson model was wrong and a new model was needed. Rutherford supposed that the positive charge, instead of being distributed through a s ...
... Most of the particles were scattered through rather small angles, but a very small fraction of them were scattered through large angles, approaching 180°. So the Thomson model was wrong and a new model was needed. Rutherford supposed that the positive charge, instead of being distributed through a s ...
Atomic Structure Video KEY
... All atoms of the same element are the same in all respects. Differing elements have atoms differing in weight. ...
... All atoms of the same element are the same in all respects. Differing elements have atoms differing in weight. ...
pwpt chemistry1
... Number of protons balanced by an equal number of negatively charged electrons ...
... Number of protons balanced by an equal number of negatively charged electrons ...
1035239Notes 4.4
... • Occurs because an atom’s nucleus is unstable or overcrowded • The atom gains stability by losing energy or emitting radiation (like a pencil tipping over) • An atom continues to be radioactive and undergoes decay until its nucleus becomes stable ...
... • Occurs because an atom’s nucleus is unstable or overcrowded • The atom gains stability by losing energy or emitting radiation (like a pencil tipping over) • An atom continues to be radioactive and undergoes decay until its nucleus becomes stable ...
Atom - MrPrimmer.com
... guidelines should be used when drawing Bohr-Rutherford diagrams: i. Determine the number of protons, neutrons and electrons the atom has. ii. Draw a small circle representing the nucleus. iii. Inside the nucleus, write the number of protons the atom has. iv. Also inside the nucleus, write the number ...
... guidelines should be used when drawing Bohr-Rutherford diagrams: i. Determine the number of protons, neutrons and electrons the atom has. ii. Draw a small circle representing the nucleus. iii. Inside the nucleus, write the number of protons the atom has. iv. Also inside the nucleus, write the number ...
Ch04Notes - Mr. Julien`s Homepage
... Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: e. Students know the nucleus of the atom is ...
... Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: e. Students know the nucleus of the atom is ...
Radioactive Decay
... short period of time, if the nucleus gets large and there is a larger number of neutrons then neutrons become more and more isolated from protons and thus can decay. If there are not enough neutrons then the protons will repel each other and result in an unstable nucleus. The nucleus can also be exc ...
... short period of time, if the nucleus gets large and there is a larger number of neutrons then neutrons become more and more isolated from protons and thus can decay. If there are not enough neutrons then the protons will repel each other and result in an unstable nucleus. The nucleus can also be exc ...
sch3u unit 1 test: matter
... a. Fluorescent lights contain fluorine gas. b. There are no gases present in fluorescent lights. c. The same elements are present in all fluorescent lights. d. Air is present in all fluorescent lights. 4. Choose the pair of elements that will form a compound with the most ionic character a. Li & O b ...
... a. Fluorescent lights contain fluorine gas. b. There are no gases present in fluorescent lights. c. The same elements are present in all fluorescent lights. d. Air is present in all fluorescent lights. 4. Choose the pair of elements that will form a compound with the most ionic character a. Li & O b ...
Prelab01
... Consider the example of the Hydrogen atom; a simplified (but very useful) model for this atom consists of a single electron going in a circular orbit around a single proton. The parameters of the Hydrogen atom are as follows: Electron mass: 9.1 x 10-31 kg; electron charge: -1.6 x 10-19 C; Proton mas ...
... Consider the example of the Hydrogen atom; a simplified (but very useful) model for this atom consists of a single electron going in a circular orbit around a single proton. The parameters of the Hydrogen atom are as follows: Electron mass: 9.1 x 10-31 kg; electron charge: -1.6 x 10-19 C; Proton mas ...
Objectives for Nuclear Chemistry
... Describe what each emission is composed of and how they differ from each other with respect to mass, charge, penetrating power, and ionizing power 5) Tell what happens to an element that undergoes alpha decay, beta decay, or gamma decay 6) Discuss the process used to separate the three types of radi ...
... Describe what each emission is composed of and how they differ from each other with respect to mass, charge, penetrating power, and ionizing power 5) Tell what happens to an element that undergoes alpha decay, beta decay, or gamma decay 6) Discuss the process used to separate the three types of radi ...
File
... ~ something with mass and volume (remember the density lab?) matter can usually be found on Earth as a solid, liquid, or a gas ...
... ~ something with mass and volume (remember the density lab?) matter can usually be found on Earth as a solid, liquid, or a gas ...
Unit 2 Practice Exam exam_2p_08_matter
... 15. What is the most radioactive of everyday examples listed below? a. K of salt substitute, KCl. c. Ra of old, glow in the dark clocks. b. Am241 of smoke detectors. d. C14 from your body. 16. How many orbital electron pairs are present in a sulfur atom? a) 16 b) 8 c) 7 d) 9 17. The kernel of magnes ...
... 15. What is the most radioactive of everyday examples listed below? a. K of salt substitute, KCl. c. Ra of old, glow in the dark clocks. b. Am241 of smoke detectors. d. C14 from your body. 16. How many orbital electron pairs are present in a sulfur atom? a) 16 b) 8 c) 7 d) 9 17. The kernel of magnes ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.