Chapter 2 - My Teacher Site
... When an electron loses energy, it “falls back” to a shell closer to the nucleus, with the lost energy usually released to the environment as heat ...
... When an electron loses energy, it “falls back” to a shell closer to the nucleus, with the lost energy usually released to the environment as heat ...
lhc
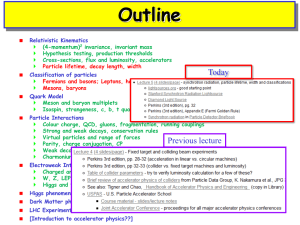
... cells (10-6 m) which were first probed by microscopes. Hence accelerators are like microscopes into the tiny world of fundamental particles and their interactions. They help us find answers to questions such as, when and how the Universe was formed, and what it is made of. What do we know about our ...
... cells (10-6 m) which were first probed by microscopes. Hence accelerators are like microscopes into the tiny world of fundamental particles and their interactions. They help us find answers to questions such as, when and how the Universe was formed, and what it is made of. What do we know about our ...
Physical Science - The Camphor Tree
... In fission, a big nucleus splits into two or more smaller nuclei. In fusion, two small nuclei are smashed together and form a new, bigger nucleus. ...
... In fission, a big nucleus splits into two or more smaller nuclei. In fusion, two small nuclei are smashed together and form a new, bigger nucleus. ...
Course Syllabus - Honors Chemistry
... reactivity with other elements in the table. h.* The experimental basis for Thomson’s discovery of the electron, Rutherford’s nuclear atom, Millikan’s oil drop experiment, and Einstein’s explanation of the photoelectric effect. i.* The development of the quantum theory of atomic structure and the hi ...
... reactivity with other elements in the table. h.* The experimental basis for Thomson’s discovery of the electron, Rutherford’s nuclear atom, Millikan’s oil drop experiment, and Einstein’s explanation of the photoelectric effect. i.* The development of the quantum theory of atomic structure and the hi ...
Chemistry-5th-Edition-Brady-Solution-Manual
... Nonmetals are more frequently found in compounds because of the large variety of ways they may combine. A particularly illustrative example is the combination of carbon, a nonmetal, with other elements. So many compounds are possible that there is one entire area of chemistry devoted to the study of ...
... Nonmetals are more frequently found in compounds because of the large variety of ways they may combine. A particularly illustrative example is the combination of carbon, a nonmetal, with other elements. So many compounds are possible that there is one entire area of chemistry devoted to the study of ...
Chemistry-Chapter 2 Lecture Notes Page
... positive H and two other atoms (slightly negative O or N) - Easily broken by Temp or pH - Found in: H2O, Proteins, Nucleic Acids ...
... positive H and two other atoms (slightly negative O or N) - Easily broken by Temp or pH - Found in: H2O, Proteins, Nucleic Acids ...
Field and particle pictures advance notice article - Specimen
... mainly to cosmic rays. Clyde Cowan later said, ‘It is easy to shield out the noise men make, but impossible to shut out the cosmos. Neutrons and gamma rays from the reactor, which we had 60 feared most, were stopped in our thick walls of paraffin, borax and lead, but the cosmic ray mesons penetrated ...
... mainly to cosmic rays. Clyde Cowan later said, ‘It is easy to shield out the noise men make, but impossible to shut out the cosmos. Neutrons and gamma rays from the reactor, which we had 60 feared most, were stopped in our thick walls of paraffin, borax and lead, but the cosmic ray mesons penetrated ...
2. NH3 - Huffman Chemistry Website!
... (Example: Noble gases – Elements with the outermost s and P sublevels are filled.) Transition metals – Inner transition metals – Representative elements – Alkali metals Alkaline earth metals Halogens Groups Periods ...
... (Example: Noble gases – Elements with the outermost s and P sublevels are filled.) Transition metals – Inner transition metals – Representative elements – Alkali metals Alkaline earth metals Halogens Groups Periods ...
Advances in Effective Field Theories
... The strong interaction described by quantum chromodynamics is responsible for binding neutrons and protons into nuclei and for the many facets of nuclei and dense matter in astrophysics. Combined with the electroweak interaction, it determines the properties of all nuclei in ...
... The strong interaction described by quantum chromodynamics is responsible for binding neutrons and protons into nuclei and for the many facets of nuclei and dense matter in astrophysics. Combined with the electroweak interaction, it determines the properties of all nuclei in ...
Fission and Fusion
... • Nuclear reactions produce tremendous amounts of usable heat energy. • Fission is the breaking up of an unstable uranium atom. • Fission is easier to start and control than fusion, but produces less energy and generates highly radioactive waste. • In uncontrolled fission nuclear chain reactions occ ...
... • Nuclear reactions produce tremendous amounts of usable heat energy. • Fission is the breaking up of an unstable uranium atom. • Fission is easier to start and control than fusion, but produces less energy and generates highly radioactive waste. • In uncontrolled fission nuclear chain reactions occ ...
An element`s properties depend on the structure of its atoms
... • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of pote ...
... • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of pote ...
The Chemical Context of Life PPT
... On to the electron cloud… • The Energy Levels of Electrons • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in the ...
... On to the electron cloud… • The Energy Levels of Electrons • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in the ...
The Chemical Context of Life
... On to the electron cloud… • The Energy Levels of Electrons • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in the ...
... On to the electron cloud… • The Energy Levels of Electrons • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in the ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.