Radiographers
... Isotope: Atoms having the same atomic number “Z” but different atomic mass “A”. All Isotopes have the same chemical properties because they have the same number of protons. Ex: I , I , I , I . Iodine Isotopes. Types of Isotopes: Radioactive Isotopes: they emit radiation. Such as Alpha, beta an ...
... Isotope: Atoms having the same atomic number “Z” but different atomic mass “A”. All Isotopes have the same chemical properties because they have the same number of protons. Ex: I , I , I , I . Iodine Isotopes. Types of Isotopes: Radioactive Isotopes: they emit radiation. Such as Alpha, beta an ...
Chapter 5: Electrons in Atoms 1 Section 5.1: Light and Quantized
... Energy of a photon of light must have a certain minimum value (threshold) to cause the ejection of a photoelectron o For photoelectric effect to occur, the photon must have the minimum amount of energy required to free an electron from the atoms of the metal. ...
... Energy of a photon of light must have a certain minimum value (threshold) to cause the ejection of a photoelectron o For photoelectric effect to occur, the photon must have the minimum amount of energy required to free an electron from the atoms of the metal. ...
Microsoft Word Format - University of Toronto Physics
... Lastly, we note that the target electrons are not free as has been assumed so far, but bound in atoms, molecules, often in condensed matter. However, the low-Z elements do not have tightly bound electrons. For example, the average K-shell binding energy of oxygen electrons is 0.7 keV, which is very ...
... Lastly, we note that the target electrons are not free as has been assumed so far, but bound in atoms, molecules, often in condensed matter. However, the low-Z elements do not have tightly bound electrons. For example, the average K-shell binding energy of oxygen electrons is 0.7 keV, which is very ...
Chapter 6. Electronic Structure of Atoms
... Some phenomena cannot be explained using a wave model of light. •Blackbody radiation is the emission of light from hot objects. •The photoelectric effect is the emission of electrons from metal surfaces on which light shines. •Emission spectra are the emissions of light from electronically excited g ...
... Some phenomena cannot be explained using a wave model of light. •Blackbody radiation is the emission of light from hot objects. •The photoelectric effect is the emission of electrons from metal surfaces on which light shines. •Emission spectra are the emissions of light from electronically excited g ...
Atomic Structure
... 1. Electrons are particles that travel in circular orbits about the nucleus. 2. Orbits are quantized - only certain ones are allowed- the lowest one is called the ground state. 3. Energy is absorbed going from lower to higher orbits (lower ones are closer to the nucleus, higher ones are farther away ...
... 1. Electrons are particles that travel in circular orbits about the nucleus. 2. Orbits are quantized - only certain ones are allowed- the lowest one is called the ground state. 3. Energy is absorbed going from lower to higher orbits (lower ones are closer to the nucleus, higher ones are farther away ...
Electricity and Magnetism
... • Rule 1: Like charges repel one another • Rule 2: Unlike charges attract one another ...
... • Rule 1: Like charges repel one another • Rule 2: Unlike charges attract one another ...
Chem312 Au03 Problem Set 4
... of one electron from the t2g set of orbitals to the t2g eg set. In a diagram like the one at right, add ground state excited state electrons to represent the ground state and the lowest energy excited state. When you put the electrons in, you should follow Hund’s rule, that a state is lower in energ ...
... of one electron from the t2g set of orbitals to the t2g eg set. In a diagram like the one at right, add ground state excited state electrons to represent the ground state and the lowest energy excited state. When you put the electrons in, you should follow Hund’s rule, that a state is lower in energ ...
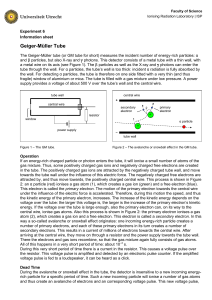
6 Geiger-Müller Tube - Ioniserende Stralen Practicum
... If an energy-rich charged particle or photon enters the tube, it will ionise a small number of atoms of the gas mixture. Thus, some positively charged gas ions and negatively charged free electrons are created in the tube. The positively charged gas ions are attracted by the negatively charged tube ...
... If an energy-rich charged particle or photon enters the tube, it will ionise a small number of atoms of the gas mixture. Thus, some positively charged gas ions and negatively charged free electrons are created in the tube. The positively charged gas ions are attracted by the negatively charged tube ...
18 Multi-electron Atom
... Thus, the magnitude magnetic moment of the electron is fixed, and the direction it points is quantized and can take on one of two values. Since any component of L has 2l + 1 eigenvalues we would expect that the magnetic moment to have 2l + 1 eigenvalues. However, experimentally only two distinct tr ...
... Thus, the magnitude magnetic moment of the electron is fixed, and the direction it points is quantized and can take on one of two values. Since any component of L has 2l + 1 eigenvalues we would expect that the magnetic moment to have 2l + 1 eigenvalues. However, experimentally only two distinct tr ...
Chemistry Lesson Plans #12
... wavelengths – these are the Lyman series If the electron is dropping from the 3-7 energy levels to n=2, then emission spectra is produced in the visible wavelengths – these are the Balmer series If the electron is dropping from the 4-7 energy levels to n=3, then emission spectra is produced in the i ...
... wavelengths – these are the Lyman series If the electron is dropping from the 3-7 energy levels to n=2, then emission spectra is produced in the visible wavelengths – these are the Balmer series If the electron is dropping from the 4-7 energy levels to n=3, then emission spectra is produced in the i ...
subatomic-particles
... quantum scale matter and energy behave very differently from what much of everyday experience would lead us to expect. The idea of a particle underwent serious rethinking when experiments showed that light could behave like a stream of particles (called photons) as well as exhibit wave-like properti ...
... quantum scale matter and energy behave very differently from what much of everyday experience would lead us to expect. The idea of a particle underwent serious rethinking when experiments showed that light could behave like a stream of particles (called photons) as well as exhibit wave-like properti ...
Aulenbacher_EUCARD_coordination_meeting3_talk
... „MIMIC“ impact of photon beam by ionization loss due to focussed electron beam at several MeV. High repetition rate possible at c.w. electron accelerators. 1 day of operation at MAMI mimicks 1 year operation at the ILC. (first experiment on Titanium alloy 2/2016) ...
... „MIMIC“ impact of photon beam by ionization loss due to focussed electron beam at several MeV. High repetition rate possible at c.w. electron accelerators. 1 day of operation at MAMI mimicks 1 year operation at the ILC. (first experiment on Titanium alloy 2/2016) ...
Quantum Theory
... Objects do not always have specific properties until they are interacted with; the properties hang in some sort of limbo. ...
... Objects do not always have specific properties until they are interacted with; the properties hang in some sort of limbo. ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.