New Theories of Gravitation and Particle Model Chongxi Yu
... matter[3] and dark energy[4], both of them are never found. All current theories of gravitation cannot explain flyby anomaly[5], anomalous increase of the astronomical unit, extra energetic photons, and extra massive hydrogen clouds[6]. Although the standard model has demonstrated successes in provi ...
... matter[3] and dark energy[4], both of them are never found. All current theories of gravitation cannot explain flyby anomaly[5], anomalous increase of the astronomical unit, extra energetic photons, and extra massive hydrogen clouds[6]. Although the standard model has demonstrated successes in provi ...
Study of the Neutron Detection Efficiency of the CLAS12 Detector
... photo multiplier tubes (PMT). They are used to help distinguish similar particles from one another for example, a π − and an electron. The problem is both particles have a negative charge so both bend outward in the magnetic field; however, the π − is a more massive particle so it moves slower than ...
... photo multiplier tubes (PMT). They are used to help distinguish similar particles from one another for example, a π − and an electron. The problem is both particles have a negative charge so both bend outward in the magnetic field; however, the π − is a more massive particle so it moves slower than ...
Chapter 1 Principles of Semiconductor Physics - Wiley-VCH
... According to Eq. (1.14), the negative curvature of the valence band would mean a negative electron mass, which is physically not acceptable. It has therefore been concluded that occupied orbitals in the valence band correspond to holes. A hole acts in an applied electric or magnetic field as though i ...
... According to Eq. (1.14), the negative curvature of the valence band would mean a negative electron mass, which is physically not acceptable. It has therefore been concluded that occupied orbitals in the valence band correspond to holes. A hole acts in an applied electric or magnetic field as though i ...
Chapter 5 - CARSON`S CHEMISTRY CLASS
... greater amount of energy, and emits different colors of light. These different colors correspond to different frequencies and wavelengths. The wave model could not explain the emission of these different wavelengths of light at different temperatures. In 1900, the German physicist Max Planck (1858–1 ...
... greater amount of energy, and emits different colors of light. These different colors correspond to different frequencies and wavelengths. The wave model could not explain the emission of these different wavelengths of light at different temperatures. In 1900, the German physicist Max Planck (1858–1 ...
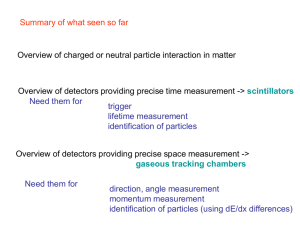
gaseous tracking chambers
... cannot be made by directly depositing metal on the semiconductor (else a rectifing junction extending into the semiconductor is formed). So heavily doped layers of n+ or p+ are used between the semiconductor and the ...
... cannot be made by directly depositing metal on the semiconductor (else a rectifing junction extending into the semiconductor is formed). So heavily doped layers of n+ or p+ are used between the semiconductor and the ...
Drift Speed Questions - G482
... The current I flowing through a conductor of cross-sectional area A is given by the formula I = nAQ where Q is the charge on a charge carrier. Give the meanings of n and . n ............................................................................................................................ ...
... The current I flowing through a conductor of cross-sectional area A is given by the formula I = nAQ where Q is the charge on a charge carrier. Give the meanings of n and . n ............................................................................................................................ ...
Chapter 5 pdf
... greater amount of energy, and emits different colors of light. These different colors correspond to different frequencies and wavelengths. The wave model could not explain the emission of these different wavelengths of light at different temperatures. In 1900, the German physicist Max Planck (1858–1 ...
... greater amount of energy, and emits different colors of light. These different colors correspond to different frequencies and wavelengths. The wave model could not explain the emission of these different wavelengths of light at different temperatures. In 1900, the German physicist Max Planck (1858–1 ...
Answers
... b) Yes, a negative particle has been produced that moves up and to the left. c) Yes, a positive particle has been produced that moves up and to the left. d) Yes, a neutral particle has been produced that moves up and to the left. Momentum is always conserved. There must be another moving particle. W ...
... b) Yes, a negative particle has been produced that moves up and to the left. c) Yes, a positive particle has been produced that moves up and to the left. d) Yes, a neutral particle has been produced that moves up and to the left. Momentum is always conserved. There must be another moving particle. W ...
The Two Slit Experiment
... conclusions reached are what would be expected on the basis of what is now known about quantum mechanics from a multitude of other experiments. Thus, this largely hypothetical experiment (otherwise known as a thought experiment or gedanken experiment) serves to illustrate the kind of behaviour that ...
... conclusions reached are what would be expected on the basis of what is now known about quantum mechanics from a multitude of other experiments. Thus, this largely hypothetical experiment (otherwise known as a thought experiment or gedanken experiment) serves to illustrate the kind of behaviour that ...
Your Paper`s Title Starts Here:
... Abstract. The paper examines the role and influence of transient phenomenon on the transport efficiency of high current (5-20 kA) low energy (tens of keV) electron beams in a pipe filled with low-pressure plasma in an external magnetic field. The research is based on the self-consistent mathematical ...
... Abstract. The paper examines the role and influence of transient phenomenon on the transport efficiency of high current (5-20 kA) low energy (tens of keV) electron beams in a pipe filled with low-pressure plasma in an external magnetic field. The research is based on the self-consistent mathematical ...
Chapter 2 ATOMIC THEORY - Beck-Shop
... subatomic (nuclear) physics. In November 1895, Wilhelm Roentgen (1845– 1923) discovered a new type of radiation called X-rays, and their ability to penetrate highly dense materials. Soon after the discovery of X-rays, Henri Becquerel (1852–1908) showed that certain materials emit similar rays indepe ...
... subatomic (nuclear) physics. In November 1895, Wilhelm Roentgen (1845– 1923) discovered a new type of radiation called X-rays, and their ability to penetrate highly dense materials. Soon after the discovery of X-rays, Henri Becquerel (1852–1908) showed that certain materials emit similar rays indepe ...
Chapter 2 ATOMIC THEORY
... subatomic (nuclear) physics. In November 1895, Wilhelm Roentgen (1845– 1923) discovered a new type of radiation called X-rays, and their ability to penetrate highly dense materials. Soon after the discovery of X-rays, Henri Becquerel (1852–1908) showed that certain materials emit similar rays indepe ...
... subatomic (nuclear) physics. In November 1895, Wilhelm Roentgen (1845– 1923) discovered a new type of radiation called X-rays, and their ability to penetrate highly dense materials. Soon after the discovery of X-rays, Henri Becquerel (1852–1908) showed that certain materials emit similar rays indepe ...
Activity 2 Tiny and Indivisible
... experiment that there is a basic unit of charge. J.J.Thomson had “discovered” the electron, a tiny negatively charged particle, about ten years before Millikan’s experiment. He did this by analyzing electron beams in a tube very similar to the tube where electrons travel in your television. It is th ...
... experiment that there is a basic unit of charge. J.J.Thomson had “discovered” the electron, a tiny negatively charged particle, about ten years before Millikan’s experiment. He did this by analyzing electron beams in a tube very similar to the tube where electrons travel in your television. It is th ...
ABSTRACT - University of Richmond
... The following layer is made of plastic scintillators to determine time of flight (ToF) and hence velocity when combined with the path length from the trajectory measured with the drift chambers. The scintillators are located 3 meters from the target, and contain 300 bars (2”x 6”) ranging in length f ...
... The following layer is made of plastic scintillators to determine time of flight (ToF) and hence velocity when combined with the path length from the trajectory measured with the drift chambers. The scintillators are located 3 meters from the target, and contain 300 bars (2”x 6”) ranging in length f ...
An Electrostatic Solution for the Gravity Force and the Value of G
... V / r ‘fields’, the two electrons for the model calculation are assumed to be positioned in Hydrogen atoms. Although the "daraf" ( 1 / C unit of elastance S for a capacitor with "farad" unit of capacitance C ) is not used very often, it is used in this paper for V / S voltage gradients and for QV / ...
... V / r ‘fields’, the two electrons for the model calculation are assumed to be positioned in Hydrogen atoms. Although the "daraf" ( 1 / C unit of elastance S for a capacitor with "farad" unit of capacitance C ) is not used very often, it is used in this paper for V / S voltage gradients and for QV / ...
Particle Detectors - Forschungszentrum Jülich
... The "drift" in the name of this chamber refers to the time it takes electrons to drift to the nearest sense wire from the place where the high-energy particle ionized an atom. Any three sense wires are only nearby in one place so a set of "hits" on these three fix a particle track in this region. By ...
... The "drift" in the name of this chamber refers to the time it takes electrons to drift to the nearest sense wire from the place where the high-energy particle ionized an atom. Any three sense wires are only nearby in one place so a set of "hits" on these three fix a particle track in this region. By ...
Ch 19all 20.2
... field inside a wire? • What is the role of the battery in a circuit? In an electric circuit the system does not reach equilibrium! Steady state and static equilibrium Static equilibrium: • no charges are moving Steady state (Dynamic Equilibrium): • charges are moving • their velocities at any locati ...
... field inside a wire? • What is the role of the battery in a circuit? In an electric circuit the system does not reach equilibrium! Steady state and static equilibrium Static equilibrium: • no charges are moving Steady state (Dynamic Equilibrium): • charges are moving • their velocities at any locati ...
Radioactive Decay
... Note that β+ decay and electron capture produce the same products. Electron capture can sometimes (but not often) occur without β+ decay. However, β+ decay is always accompanied by electron capture. Atoms are likely to undergo electron capture (and usually also β+ decay) if they have too many proton ...
... Note that β+ decay and electron capture produce the same products. Electron capture can sometimes (but not often) occur without β+ decay. However, β+ decay is always accompanied by electron capture. Atoms are likely to undergo electron capture (and usually also β+ decay) if they have too many proton ...
Chapter 21. Electric Charge
... negative; a proton has a positive charge, and an electron has a negative charge. The SI unit for measuring the magnitude of electric charge is the coulomb (C). The electric charge is said to be quantized. The smallest amount of free charge is e=1.6×10-19 C. Any electric charge, q, occurs as integer ...
... negative; a proton has a positive charge, and an electron has a negative charge. The SI unit for measuring the magnitude of electric charge is the coulomb (C). The electric charge is said to be quantized. The smallest amount of free charge is e=1.6×10-19 C. Any electric charge, q, occurs as integer ...
2.8 M - Thierry Karsenti
... technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. ...
... technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.