1 - barnes report
... 1. Units for length and energy A convenient unit of length for description of solids is the nanometer (nm), which is the order of magnitude of a typical distance between atoms. (Actual sizes are between 0.1 nm and 1.0 nm. Many older texts use the Angstrom = 0.1 nm.) A convenient unit of energy is t ...
... 1. Units for length and energy A convenient unit of length for description of solids is the nanometer (nm), which is the order of magnitude of a typical distance between atoms. (Actual sizes are between 0.1 nm and 1.0 nm. Many older texts use the Angstrom = 0.1 nm.) A convenient unit of energy is t ...
MYP Chemistry: Final Review
... What is the electromagnetic (EM) spectrum? What is the highest energy wave? Lowest? The range of all known light, the lowest being radio waves and the highest being gamma rays. Visible light (ROYGBV) is a tiny portion of the spectrum in the middle ...
... What is the electromagnetic (EM) spectrum? What is the highest energy wave? Lowest? The range of all known light, the lowest being radio waves and the highest being gamma rays. Visible light (ROYGBV) is a tiny portion of the spectrum in the middle ...
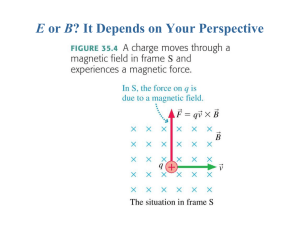
E or B? It Depends on Your Perspective
... Maxwell, using his equations of the electromagnetic field, was the first to understand that light is an oscillation of the electromagnetic field. Maxwell was able to predict that • Electromagnetic waves can exist at any frequency, not just at the frequencies of visible light. This prediction was the ...
... Maxwell, using his equations of the electromagnetic field, was the first to understand that light is an oscillation of the electromagnetic field. Maxwell was able to predict that • Electromagnetic waves can exist at any frequency, not just at the frequencies of visible light. This prediction was the ...
AP Physics B Exam Cram Sheet - Mater Academy Lakes High School
... 24. The gravitational force (and the resulting acceleration) due to a planet varies directly with the distance from the center of the planet when the object is INSIDE the planet. 25. The gravitational force (and the resulting acceleration) due to a planet varies inversely with the square of the dis ...
... 24. The gravitational force (and the resulting acceleration) due to a planet varies directly with the distance from the center of the planet when the object is INSIDE the planet. 25. The gravitational force (and the resulting acceleration) due to a planet varies inversely with the square of the dis ...
AP Physics B Exam Cram Sheet
... 24. The gravitational force (and the resulting acceleration) due to a planet varies directly with the distance from the center of the planet when the object is INSIDE the planet. 25. The gravitational force (and the resulting acceleration) due to a planet varies inversely with the square of the dis ...
... 24. The gravitational force (and the resulting acceleration) due to a planet varies directly with the distance from the center of the planet when the object is INSIDE the planet. 25. The gravitational force (and the resulting acceleration) due to a planet varies inversely with the square of the dis ...
Class16review
... Electric fields are created when positive charges and negative charges are separated A uniform electric field existing over a region sets up a potential difference between points in that region: DV=EDx, where Dx is the distance along a field line. If I apply a potential difference across a ...
... Electric fields are created when positive charges and negative charges are separated A uniform electric field existing over a region sets up a potential difference between points in that region: DV=EDx, where Dx is the distance along a field line. If I apply a potential difference across a ...
CHAPTER 2 Introduction to Quantum Mechanics
... At a constant incident intensity, the maximum kinetic energy of the photoelectron varies linearly with frequency with a limiting frequency v = v0, below which no photoelectron is produced. ...
... At a constant incident intensity, the maximum kinetic energy of the photoelectron varies linearly with frequency with a limiting frequency v = v0, below which no photoelectron is produced. ...
HW-1-Ch1-Atomic-structure-W16
... 35. Penetration & Shielding of an Electron in multi-electron atom and how does it affect the filling order as given by “Building Up” principle? ...
... 35. Penetration & Shielding of an Electron in multi-electron atom and how does it affect the filling order as given by “Building Up” principle? ...
effective nuclear charge
... react with hydrogen to form HX, acids melting point and boiling point increases down the column density increases down the column ◦ in general, the increase in mass is greater than the increase in volume ...
... react with hydrogen to form HX, acids melting point and boiling point increases down the column density increases down the column ◦ in general, the increase in mass is greater than the increase in volume ...