next article
... where the cj are constants, for substitution of a typical term of (21) reduces (20) to an expression differing from (17) only by a constant factor. Now by (19) the term proportional to cj in (21) simply adds to (15) very approximately the change cjihb( ,IeS/ih)/baj which results in (15) if a, is alt ...
... where the cj are constants, for substitution of a typical term of (21) reduces (20) to an expression differing from (17) only by a constant factor. Now by (19) the term proportional to cj in (21) simply adds to (15) very approximately the change cjihb( ,IeS/ih)/baj which results in (15) if a, is alt ...
Physical Chemistry Born`s interpretation of the wave function
... can be normalized and represent probability.) Single-valued (so that the probability at any point is unique) Continuous at all points in space. First derivative must be continuous at all points where the potential is continuous. ...
... can be normalized and represent probability.) Single-valued (so that the probability at any point is unique) Continuous at all points in space. First derivative must be continuous at all points where the potential is continuous. ...
The One-Dimensional Finite-Difference Time
... and the barrier thickness is set to T = 0.25 Å, or 25 grid points. The simulation domain consists of L = 3000 grid points, and the simulation was run for N = 12, 000 time steps. The figure shows four snapshots of the simulation as it progressed in time. As the particle collides with the potential b ...
... and the barrier thickness is set to T = 0.25 Å, or 25 grid points. The simulation domain consists of L = 3000 grid points, and the simulation was run for N = 12, 000 time steps. The figure shows four snapshots of the simulation as it progressed in time. As the particle collides with the potential b ...
Solution Set 8 Worldsheet perspective on CY compactification
... solutions other than φi = 0, ∀i) then the second term in the F-term potential forces P to vanish. Then the first term (which much vanish independently of the second since they are both positive) forces the massless modes onto the locus G5 = 0 which is the quintic. Relate the moduli of the quintic t ...
... solutions other than φi = 0, ∀i) then the second term in the F-term potential forces P to vanish. Then the first term (which much vanish independently of the second since they are both positive) forces the massless modes onto the locus G5 = 0 which is the quintic. Relate the moduli of the quintic t ...
pdf file
... deflected upwards (that is their spin along the y-axis is + h /2). (a) What percentage of those would then have a spin of - h /2 when they traverse a Stern-Gerlach device oriented along the zdirection? (b) Now, of those particles, what percentage will have a spin of + h /2 when they traverse a third ...
... deflected upwards (that is their spin along the y-axis is + h /2). (a) What percentage of those would then have a spin of - h /2 when they traverse a Stern-Gerlach device oriented along the zdirection? (b) Now, of those particles, what percentage will have a spin of + h /2 when they traverse a third ...
Document
... types of inversion formulas that reconstruct a centrosymmetric field V(Y) from the deflection angle x of the particle: a t a given particle energy E (Ref. I), a t a given angular momentum I (Ref. 2) and a t a given impact parameter b. We a s s u m e hereafter V(r) is a r e pulsive field, i.e., V> 0, ...
... types of inversion formulas that reconstruct a centrosymmetric field V(Y) from the deflection angle x of the particle: a t a given particle energy E (Ref. I), a t a given angular momentum I (Ref. 2) and a t a given impact parameter b. We a s s u m e hereafter V(r) is a r e pulsive field, i.e., V> 0, ...
MODULE 1
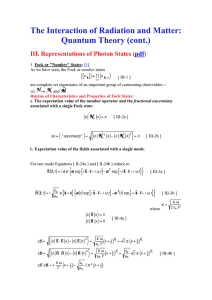
... In quantum theory the specification of the state of a system at a given time is provided by its wavefunction (Postulate 1). In order to predict the state of a sub-microscopic system at another time we need a quantum equivalent of Newton’s law that tells us how the wavefunction changes with time. ...
... In quantum theory the specification of the state of a system at a given time is provided by its wavefunction (Postulate 1). In order to predict the state of a sub-microscopic system at another time we need a quantum equivalent of Newton’s law that tells us how the wavefunction changes with time. ...