Terminology 1
... Shows which elements are present and the simplest whole number ratio of their atoms, but not necessarily the actual number of atoms present in the molecule. (simplest chemical ...
... Shows which elements are present and the simplest whole number ratio of their atoms, but not necessarily the actual number of atoms present in the molecule. (simplest chemical ...
Quantum physics
... • Photocurrent I = (n/t)e, where (n/t) = rate of emission of electrons • Why rate of emission of electrons << rate of incidence of photons {for f>f0}: • Not every photon would collide with an electron; most are reflected by the metal or miss hitting any electron. • On the way out to the metal surfac ...
... • Photocurrent I = (n/t)e, where (n/t) = rate of emission of electrons • Why rate of emission of electrons << rate of incidence of photons {for f>f0}: • Not every photon would collide with an electron; most are reflected by the metal or miss hitting any electron. • On the way out to the metal surfac ...
The Chemical Context of Life
... • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number 原子序 is the number of protons in its nucleus (ex. 2He) • An element’s mass number 質量數 is the sum of protons + neutrons in the nucleus (ex. 24He or 1123Na) • Atomic mass 原子量, the atom’s total mass, can ...
... • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number 原子序 is the number of protons in its nucleus (ex. 2He) • An element’s mass number 質量數 is the sum of protons + neutrons in the nucleus (ex. 24He or 1123Na) • Atomic mass 原子量, the atom’s total mass, can ...
Electron Spin I - Rutgers Physics
... The z in the upper left corner indicates that the magnetic field gradient is oriented in the negative z-direction. This splits the electrons into two beams in z with the upper beam corresponding to electrons with spin-up in the z-direction and the lower beam to electrons with spin-down in the z-dire ...
... The z in the upper left corner indicates that the magnetic field gradient is oriented in the negative z-direction. This splits the electrons into two beams in z with the upper beam corresponding to electrons with spin-up in the z-direction and the lower beam to electrons with spin-down in the z-dire ...
File
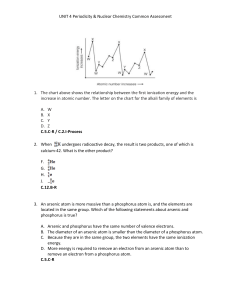
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
Atomic Structure Lecture 7 - Introduction Lecture 7
... The wave function, !, is also called an atomic orbital. • There is a different wave function for each of the different energy states that an electron can have in an atom While the wave function, !, has no physical meaning, the square of the wave function, !2, is does. • !2 is called the probability ...
... The wave function, !, is also called an atomic orbital. • There is a different wave function for each of the different energy states that an electron can have in an atom While the wave function, !, has no physical meaning, the square of the wave function, !2, is does. • !2 is called the probability ...
Introduction to Computational Chemistry
... physical measurements with respect to the behaviour of the molecular system under investigation. This could possibly be an NMR or IR spectrum, the measurement of a pKa value, a kinetic measurement or a ...
... physical measurements with respect to the behaviour of the molecular system under investigation. This could possibly be an NMR or IR spectrum, the measurement of a pKa value, a kinetic measurement or a ...
File
... •Crystal Field Theory - Describes bonding in Metal Complexes • Basic Assumption in CFT: ...
... •Crystal Field Theory - Describes bonding in Metal Complexes • Basic Assumption in CFT: ...
Unit B: Matter and Chemical Change
... Matter: anything that occupies space (volume) and contains mass. Pure Substance: made of only one kind of matter and has a unique set of properties (chemical and physical). e.g., mercury (element) and sugar (compound). Element: a material that cannot be broken down into any simpler substance. e.g. ...
... Matter: anything that occupies space (volume) and contains mass. Pure Substance: made of only one kind of matter and has a unique set of properties (chemical and physical). e.g., mercury (element) and sugar (compound). Element: a material that cannot be broken down into any simpler substance. e.g. ...
The Photoelectric Effect, work function
... equation of line: y = mx + b :: Ek = hf – W (we want W to be a positive number, so we must use a – sign) W is called the Work Function. It is the amount of energy needed to blast the electrons out of the metal (break the attraction between the electrons and the rest of the atom). This energy comes f ...
... equation of line: y = mx + b :: Ek = hf – W (we want W to be a positive number, so we must use a – sign) W is called the Work Function. It is the amount of energy needed to blast the electrons out of the metal (break the attraction between the electrons and the rest of the atom). This energy comes f ...
Chapter 6 Electronic Structure of Atoms
... Energies of Orbitals • As the number of __________________ increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms ...
... Energies of Orbitals • As the number of __________________ increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms ...
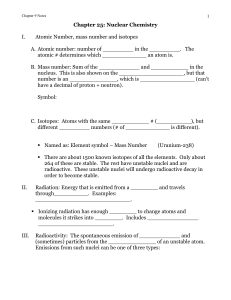
Chapter 9: Nuclear Chemistry
... B. Beta ( ) emission” particles that are _____________ emitted at _________ the speed of light. _______________ charged. ________ penetrating than alpha particles. There are also ______ particles (_______________) that are identical to electrons but have a ____ charge. Symbols: C. Gamma ( ) emission ...
... B. Beta ( ) emission” particles that are _____________ emitted at _________ the speed of light. _______________ charged. ________ penetrating than alpha particles. There are also ______ particles (_______________) that are identical to electrons but have a ____ charge. Symbols: C. Gamma ( ) emission ...
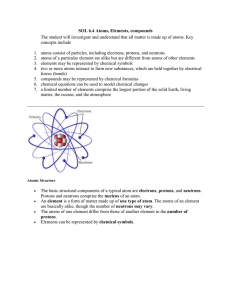
Atoms, Elements, Compounds File
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
Department of Physical Sciences (Physics)
... (iv) Orbiting satellites and spacecraft can become charged because the light from the sun ejects electrons from their outer surface and they must be designed to minimise this effect. If the skin is coated with Ni which has a work function of 4.87eV, calculate the longest wavelength of the incident s ...
... (iv) Orbiting satellites and spacecraft can become charged because the light from the sun ejects electrons from their outer surface and they must be designed to minimise this effect. If the skin is coated with Ni which has a work function of 4.87eV, calculate the longest wavelength of the incident s ...
Optically polarized atoms_ch_2
... This is like planetary spin and orbital motion On a short time scale, conservation of individual angular momenta can be a good approximation l and s are coupled via spin-orbit interaction: interaction of the motional magnetic field in the electron’s frame with μs Energy shift depends on relative ori ...
... This is like planetary spin and orbital motion On a short time scale, conservation of individual angular momenta can be a good approximation l and s are coupled via spin-orbit interaction: interaction of the motional magnetic field in the electron’s frame with μs Energy shift depends on relative ori ...
CHM 1025 Chapter 9 web
... contained protons. He could account for the charge of the nucleus, but the mass of was too large for the number of protons. • Protons and neutrons make up most of the mass of the atom and are in the nucleus. • Electrons are very light and are flying around outside the nucleus. C. Gambino ...
... contained protons. He could account for the charge of the nucleus, but the mass of was too large for the number of protons. • Protons and neutrons make up most of the mass of the atom and are in the nucleus. • Electrons are very light and are flying around outside the nucleus. C. Gambino ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.