using standard syste - the Max Planck Institute for the Physics of
... the classical equations of motion and the existence of chaos in the classical dynamics of the three-body system. Two-electron atoms have played an important role in the development of theoretical physics in this century: They were a catalyst for the quantum theory in the mid 1920s, since the old qua ...
... the classical equations of motion and the existence of chaos in the classical dynamics of the three-body system. Two-electron atoms have played an important role in the development of theoretical physics in this century: They were a catalyst for the quantum theory in the mid 1920s, since the old qua ...
Modeling the patterned two-dimensional electron gas: Electrostatics
... low temperature. This is a “frozen” surface. The high dielectric constant of semiconductors means that the boundary condition for this model is approximately ∂φ/∂n = 0. We assume that the layer of donors simply behaves as a fixed charge density which does not respond to the gate voltage. This implie ...
... low temperature. This is a “frozen” surface. The high dielectric constant of semiconductors means that the boundary condition for this model is approximately ∂φ/∂n = 0. We assume that the layer of donors simply behaves as a fixed charge density which does not respond to the gate voltage. This implie ...
Topic 1: Quantitative chemistry (12
... Students should be able to draw an energy level diagram, show transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible an ...
... Students should be able to draw an energy level diagram, show transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible an ...
Non-abelian quantum Hall states and fractional charges in one dimension Emma Wikberg
... The fractional quantum Hall effect has, since its discovery around 30 years ago, been a vivid field of research—both experimentally and theoretically. In this thesis we investigate certain non-abelian quantum Hall states by mapping the two-dimensional system onto a thin torus, where the problem beco ...
... The fractional quantum Hall effect has, since its discovery around 30 years ago, been a vivid field of research—both experimentally and theoretically. In this thesis we investigate certain non-abelian quantum Hall states by mapping the two-dimensional system onto a thin torus, where the problem beco ...
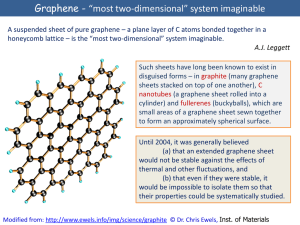
Graphene - âmost two-dimensionalâ system imaginable
... but quantum relativistic effects are usually minute in the known experimental systems that can be described accurately by the non-relativistic Schrödinger equation. Here we report an experimental study of a condensed-matter system (graphene, a single atomic layer of carbon) in which electron transpo ...
... but quantum relativistic effects are usually minute in the known experimental systems that can be described accurately by the non-relativistic Schrödinger equation. Here we report an experimental study of a condensed-matter system (graphene, a single atomic layer of carbon) in which electron transpo ...
Final Exam - KFUPM Faculty List
... Q19. Arrange the following ions: K+, S2-, Ca2+, Cl-, in order of increasing ionic radius. A) Ca2+ < K+ < Cl- < S2B) K+ < Ca2+ < Cl- < S2C) S2- < Cl- < K+ < Ca2+ D) Cl- < S2- < Ca2+ < K+ E) Ca2+ < K+ < S2- < ClIf the ions are isoelectronic then the cations are smaller and the smaller, the larger the ...
... Q19. Arrange the following ions: K+, S2-, Ca2+, Cl-, in order of increasing ionic radius. A) Ca2+ < K+ < Cl- < S2B) K+ < Ca2+ < Cl- < S2C) S2- < Cl- < K+ < Ca2+ D) Cl- < S2- < Ca2+ < K+ E) Ca2+ < K+ < S2- < ClIf the ions are isoelectronic then the cations are smaller and the smaller, the larger the ...
Topic 1: Quantitative chemistry (12
... Students should be able to draw an energy level diagram, show transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible an ...
... Students should be able to draw an energy level diagram, show transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible an ...
Non-abelian quantum Hall states and fractional charges in
... The fractional quantum Hall effect has, since its discovery around 30 years ago, been a vivid field of research—both experimentally and theoretically. In this thesis we investigate certain non-abelian quantum Hall states by mapping the two-dimensional system onto a thin torus, where the problem beco ...
... The fractional quantum Hall effect has, since its discovery around 30 years ago, been a vivid field of research—both experimentally and theoretically. In this thesis we investigate certain non-abelian quantum Hall states by mapping the two-dimensional system onto a thin torus, where the problem beco ...
Modification of the surface electronic and chemical properties of Pt
... in which the two metals function independently. These phenomena were not considered in this work. Ruban et al. studied the changes in the electronic structure of epitaxial monolayers of one metal on another metal for the late transition metals.7 In that work, the changes in electronic structure were ...
... in which the two metals function independently. These phenomena were not considered in this work. Ruban et al. studied the changes in the electronic structure of epitaxial monolayers of one metal on another metal for the late transition metals.7 In that work, the changes in electronic structure were ...
Learning Outcomes
... (b) describe, with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shells (energy levels) (Knowledge of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ...
... (b) describe, with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shells (energy levels) (Knowledge of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ...
Illumination Intensity Dependence of the Photovoltage in
... using a KG3 filter to limit the illumination to the spectrum relevant to the dye-sensitized cell. Light intensity was measured with a calibrated photodiode (UDT Sensors Inc.) by considering the emission lamp spectrum in the experimental range used (400-750 nm). Light intensity was controlled by plac ...
... using a KG3 filter to limit the illumination to the spectrum relevant to the dye-sensitized cell. Light intensity was measured with a calibrated photodiode (UDT Sensors Inc.) by considering the emission lamp spectrum in the experimental range used (400-750 nm). Light intensity was controlled by plac ...
File
... stable ns2np6 configuration. Noble gases are unreactive because they do not want to lose their stable valence electron configuration. Noble gases exist as free atoms in nature. They only exhibit London dispersion forces in the condensed phases. Because LD forces increase with size, as the noble gas ...
... stable ns2np6 configuration. Noble gases are unreactive because they do not want to lose their stable valence electron configuration. Noble gases exist as free atoms in nature. They only exhibit London dispersion forces in the condensed phases. Because LD forces increase with size, as the noble gas ...
Chemistry - talcher autonomous college
... Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle and its significance, Schrödinger’s wave equation, significance of ψ and ψ 2 . Quantum numbers and their significance. Normalized and orthogonal wave functions ...
... Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle and its significance, Schrödinger’s wave equation, significance of ψ and ψ 2 . Quantum numbers and their significance. Normalized and orthogonal wave functions ...
Interaction- and measurement-free quantum Zeno gates for universal computation
... system to remain in a decoherence-free subspace 共DFS兲 关47–49兴, and thus greatly suppresses the possibility of decoherence via spontaneous decay. Several schemes for quantum entanglement manipulation and/or gate operation via IFM have been recently proposed 关50–54兴. Utilizing the dissipative photon-a ...
... system to remain in a decoherence-free subspace 共DFS兲 关47–49兴, and thus greatly suppresses the possibility of decoherence via spontaneous decay. Several schemes for quantum entanglement manipulation and/or gate operation via IFM have been recently proposed 关50–54兴. Utilizing the dissipative photon-a ...
Department of Chemistry Second Year Syllabus
... understanding of important spectroscopic techniques used for characterisation of molecules (NMR, UV/IR and X-ray crystallography). These aims can only be achieved by continuing the combined theoretical and practical approach introduced in the first year. By the end of the year it is expected that th ...
... understanding of important spectroscopic techniques used for characterisation of molecules (NMR, UV/IR and X-ray crystallography). These aims can only be achieved by continuing the combined theoretical and practical approach introduced in the first year. By the end of the year it is expected that th ...
Electronic structure of quantum dots
... FIG. 3. Shell structure and ‘‘magic numbers’’ in finite fermion systems. Upper left, ...
... FIG. 3. Shell structure and ‘‘magic numbers’’ in finite fermion systems. Upper left, ...
Periodic models in quantum chemical simulations of F centers in
... a point with some site symmetry. A molecular defect has its own point symmetry so that the point symmetry of the whole system is determined by the common elements of two groups: the point group of the isolated molecule and the site symmetry group of the site centered on the impurity molecule. It mig ...
... a point with some site symmetry. A molecular defect has its own point symmetry so that the point symmetry of the whole system is determined by the common elements of two groups: the point group of the isolated molecule and the site symmetry group of the site centered on the impurity molecule. It mig ...
Bose-Glass Phases of Ultracold Atoms due to Cavity Backaction Hessam Habibian,
... Bragg diffraction is a manifestation of the wave properties of light and provides a criterion for the existence of long-range order in the scattering medium [1]. Bragg diffraction of light by atoms in optical lattices was measured for various geometries and settings, from gratings of laser-cooled at ...
... Bragg diffraction is a manifestation of the wave properties of light and provides a criterion for the existence of long-range order in the scattering medium [1]. Bragg diffraction of light by atoms in optical lattices was measured for various geometries and settings, from gratings of laser-cooled at ...
EGAS41
... T. Carette, M. Nemouchi, M.R. Godefroid, P. Jönsson CP 14, p74 A theoretical study of the isotope shift on electron affinity of chlorine T. Carette, M.R. Godefroid CP 15, p75 Nonresonant corrections for the optical resonance frequency measurements in hydrogen atom L. Labzowsky, G. Schedrin, D. Solo ...
... T. Carette, M. Nemouchi, M.R. Godefroid, P. Jönsson CP 14, p74 A theoretical study of the isotope shift on electron affinity of chlorine T. Carette, M.R. Godefroid CP 15, p75 Nonresonant corrections for the optical resonance frequency measurements in hydrogen atom L. Labzowsky, G. Schedrin, D. Solo ...
Full-Text PDF
... Abstract: The geometrical structures and photophysical properties of Ir(4,6-dFppy)2 (pic) (FIrpic) and its derivative (o-FIr, m-FIr, p-FIr) with dimethylamine substituted at the picolinic acid (N∧ O) ligand were fully investigated by density functional theory and time-dependent density functional th ...
... Abstract: The geometrical structures and photophysical properties of Ir(4,6-dFppy)2 (pic) (FIrpic) and its derivative (o-FIr, m-FIr, p-FIr) with dimethylamine substituted at the picolinic acid (N∧ O) ligand were fully investigated by density functional theory and time-dependent density functional th ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.