Honors Chemistry Ms. K Pages 66
... While Fred was babysitting his younger brother, Phil, he noticed that Phil was trying to stick a magnet on the screen of their black-and-white television. The magnet did not stick to the glass, but the picture seemed to be distorted. The closer he held the magnet to the screen, the more the images b ...
... While Fred was babysitting his younger brother, Phil, he noticed that Phil was trying to stick a magnet on the screen of their black-and-white television. The magnet did not stick to the glass, but the picture seemed to be distorted. The closer he held the magnet to the screen, the more the images b ...
Chapter 2
... (1+ ions only). Neon consists of three isotopes, of which neon-20 is by far the most abundant (90.48%). The mass of that isotope, to five decimal places, is 19.99244 amu on the carbon-12 scale. The number by each peak corresponds to the fraction of all Ne+ ions represented by the isotope with that m ...
... (1+ ions only). Neon consists of three isotopes, of which neon-20 is by far the most abundant (90.48%). The mass of that isotope, to five decimal places, is 19.99244 amu on the carbon-12 scale. The number by each peak corresponds to the fraction of all Ne+ ions represented by the isotope with that m ...
A critique of recent semi-classical spin-half quantum plasma theories
... [Eq.(125.7) of [15]], where σ = ± 21 . When T ≫ ~ωce , the quantum number n would be very large, and the tiny spin-dependent correction to the “orbital energy” contributed by the high n term is clearly an insignificant effect. Any semi-classical approach must require n ≫ 1 and thus cannot possibly a ...
... [Eq.(125.7) of [15]], where σ = ± 21 . When T ≫ ~ωce , the quantum number n would be very large, and the tiny spin-dependent correction to the “orbital energy” contributed by the high n term is clearly an insignificant effect. Any semi-classical approach must require n ≫ 1 and thus cannot possibly a ...
Module 6 : Light Emitting Diode
... are inexpensive, robust and have long life (the long life of an LED is primarily due to its being a cold device, i.e. its operating temperature being much lower than that of, say, an incandescent lamp), can be modulated (i.e. switched on and off) at high speeds (this property of an LED is also due t ...
... are inexpensive, robust and have long life (the long life of an LED is primarily due to its being a cold device, i.e. its operating temperature being much lower than that of, say, an incandescent lamp), can be modulated (i.e. switched on and off) at high speeds (this property of an LED is also due t ...
The method of molecular rays O S
... atom striking the surface of a hot tungsten wire (eventually oxygen-coated) goes away as an ion. By measuring the ion current outgoing from the wire we measured directly the number of atoms striking the wire. What can we conclude about the method of molecular rays from the group of experiments we ha ...
... atom striking the surface of a hot tungsten wire (eventually oxygen-coated) goes away as an ion. By measuring the ion current outgoing from the wire we measured directly the number of atoms striking the wire. What can we conclude about the method of molecular rays from the group of experiments we ha ...
Unit 3.2 worksheet 4 atomic model of matter
... Click Here - Movie Star Planet Starcoins Generator. HOW TO BECOME POPULAR ON MSP! Tips and tricks! Hope I help :)) Video Rating: / 5. Click Here - Movie Star Planet. Hi i am writing u to ask what is the state requirments while growing for person medical needs. what will make it completely legal wher ...
... Click Here - Movie Star Planet Starcoins Generator. HOW TO BECOME POPULAR ON MSP! Tips and tricks! Hope I help :)) Video Rating: / 5. Click Here - Movie Star Planet. Hi i am writing u to ask what is the state requirments while growing for person medical needs. what will make it completely legal wher ...
Discoveries: Atoms to Quarks
... are forbidden by Pauli’s exclusion principle. electron transitions from a positive-energy state to an empty negative-energy state are allowed electron disappearance. To conserve electric charge, a positive electron (positron) must disappear e+e– annihilation. electron transitions from a nega ...
... are forbidden by Pauli’s exclusion principle. electron transitions from a positive-energy state to an empty negative-energy state are allowed electron disappearance. To conserve electric charge, a positive electron (positron) must disappear e+e– annihilation. electron transitions from a nega ...
CECAM Meeting “Development of Methods for
... •primary event is followed by charge delocalization (i.e., carrier relaxation) through the anatase crystal. At low temperature, this is an anisotropic process that involves surface charge separation along the [101] direction of the anatase crystal. Carrier relaxation along the [-101] direction can b ...
... •primary event is followed by charge delocalization (i.e., carrier relaxation) through the anatase crystal. At low temperature, this is an anisotropic process that involves surface charge separation along the [101] direction of the anatase crystal. Carrier relaxation along the [-101] direction can b ...
end of year review
... fluorine (F) _____13. Which of the following could be the molecular formula of a compound that has the ...
... fluorine (F) _____13. Which of the following could be the molecular formula of a compound that has the ...
Chapter 22: Molecular Modeling Problems
... 4. Azo Dyes. Aromatic azo compounds, ArN=NAr’, are often brightly colored and used as dyes. The color arises because light absorption leading to an excited state occurs in the visible (most organic compounds do not absorb in this region). Assuming that absorption leads to promotion of an electron fr ...
... 4. Azo Dyes. Aromatic azo compounds, ArN=NAr’, are often brightly colored and used as dyes. The color arises because light absorption leading to an excited state occurs in the visible (most organic compounds do not absorb in this region). Assuming that absorption leads to promotion of an electron fr ...
CHAPTER 10 CHEMICAL BONDING II: MOLECULAR GEOMETRY
... An sp d hybridization indicates that the electron-pair arrangement about iodine is octahedral. If four fluorines are placed around iodine, the total number of valence electrons is 35. Thirty-six electrons are required to complete an octahedral electron-pair arrangement with four bonds and two lone p ...
... An sp d hybridization indicates that the electron-pair arrangement about iodine is octahedral. If four fluorines are placed around iodine, the total number of valence electrons is 35. Thirty-six electrons are required to complete an octahedral electron-pair arrangement with four bonds and two lone p ...
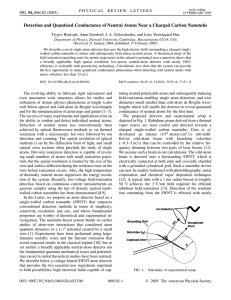
Detection and Quantized Conductance of Neutral Atoms Near a Charged... Trygve Ristroph, Anne Goodsell, J. A. Golovchenko, and Lene Vestergaard...
... and yield experimental insight into the details of the nanoscale tunneling process and the nanotube –neutral-atom interactions. The major contribution to the final kinetic energy of the outgoing ion is determined by the electrostatic potential at the position where ionization actually takes place. A ...
... and yield experimental insight into the details of the nanoscale tunneling process and the nanotube –neutral-atom interactions. The major contribution to the final kinetic energy of the outgoing ion is determined by the electrostatic potential at the position where ionization actually takes place. A ...
Class 9 CBSE Test paper Solved Chapter 3: Atoms and...
... present in definite proportions by mass”. Dalton’s atomic theory States that Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction that support Law of conservation of mass Dalton’s atomic theory States that the relative number and kinds of atoms are constant in ...
... present in definite proportions by mass”. Dalton’s atomic theory States that Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction that support Law of conservation of mass Dalton’s atomic theory States that the relative number and kinds of atoms are constant in ...
Lecture 1 Review of hydrogen atom Heavy proton (put at the origin
... Energy levels of the hydrogen atom ...
... Energy levels of the hydrogen atom ...
2008 Term 1 No 4
... the Bee, whose honeycomb hive resembles the hexagonal cathode pads in the experiment), a device much like a bubble chamber, allowing its energy and trajectory to be deduced. By taking the conservation of momentum and energy into account, the fleeting existence of the H-7 is extracted from the N-13 d ...
... the Bee, whose honeycomb hive resembles the hexagonal cathode pads in the experiment), a device much like a bubble chamber, allowing its energy and trajectory to be deduced. By taking the conservation of momentum and energy into account, the fleeting existence of the H-7 is extracted from the N-13 d ...
B.Sc. (General Sciences)
... Recapitulation of: strong, moderate and weak electrolytes, degree of ionization, factors affecting degree of ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect, Salt hydrolysis-calculation of hydrolysis constant, degree of hydr ...
... Recapitulation of: strong, moderate and weak electrolytes, degree of ionization, factors affecting degree of ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect, Salt hydrolysis-calculation of hydrolysis constant, degree of hydr ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.