What Does Quantum Mechanics Suggest About Our
... this idealistic understanding of reality. Why would they have thought this? The reason, quite simply, is that they didn’t know how to cope with the issue of quantum indeterminacy. Quantum indeterminacy is the unavoidable fact that not all quantities can simultaneously have determinate values. For e ...
... this idealistic understanding of reality. Why would they have thought this? The reason, quite simply, is that they didn’t know how to cope with the issue of quantum indeterminacy. Quantum indeterminacy is the unavoidable fact that not all quantities can simultaneously have determinate values. For e ...
on the possibility of measuring the electron spin in an
... the polarizability of electrons,and Bohr was forced to qualify his reasoning[6,i1].-The inequality(8) he endsup with in the'argument presentedhere,invoivingthe product poLr, doesnot contradict any uncertaintyrelation (as Pauli acknowledges). In fact no uncertainty relation at'all is usedjn the argum ...
... the polarizability of electrons,and Bohr was forced to qualify his reasoning[6,i1].-The inequality(8) he endsup with in the'argument presentedhere,invoivingthe product poLr, doesnot contradict any uncertaintyrelation (as Pauli acknowledges). In fact no uncertainty relation at'all is usedjn the argum ...
Chemical Bonding
... chains (which help to hold the fibers together in paper). Not surprisingly, water molecules, being polar, are also attracted to these regions and when paper is wet it loses strength because the water molecules get between the cellulose chains and weaken the attraction between them. • When water mole ...
... chains (which help to hold the fibers together in paper). Not surprisingly, water molecules, being polar, are also attracted to these regions and when paper is wet it loses strength because the water molecules get between the cellulose chains and weaken the attraction between them. • When water mole ...
Electron-electron interactions in a one-dimensional quantum
... found that in the most relevant case 共EF − E␣ Ⰶ 1兲 where the total transmission remains close to unity for a range of bandwidths, the quality of spin-filtering properties decreases substantially. Although the present paper deals with a sharp step, the present approach can be extended to steps whose ...
... found that in the most relevant case 共EF − E␣ Ⰶ 1兲 where the total transmission remains close to unity for a range of bandwidths, the quality of spin-filtering properties decreases substantially. Although the present paper deals with a sharp step, the present approach can be extended to steps whose ...
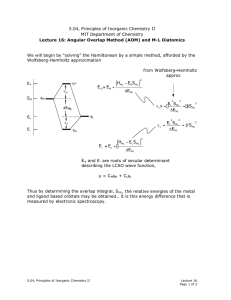
11 HC11: Molecular spectroscopy and electronic transitions van
... we can recall a number of important properties of the electronic transitions within the two-level system, represented in Figs. 10.1 and 10.2, in particular we found that: • The Einstein coefficient for stimulated absorption Bf i was proportional to the integral of the extinction coefficient over the ...
... we can recall a number of important properties of the electronic transitions within the two-level system, represented in Figs. 10.1 and 10.2, in particular we found that: • The Einstein coefficient for stimulated absorption Bf i was proportional to the integral of the extinction coefficient over the ...
Simple, accurate electrostatics-based formulas for calculating
... While these equations are classical in their overall form, the distance is quantum mechanical in its origin, as we have noted above. Thus, the addition of this finite distance to Rn in the expression above provides a kind of quantum adaptation or correction to the simple, otherwise classical electro ...
... While these equations are classical in their overall form, the distance is quantum mechanical in its origin, as we have noted above. Thus, the addition of this finite distance to Rn in the expression above provides a kind of quantum adaptation or correction to the simple, otherwise classical electro ...
3 Fundamentals of Planetary Materials
... has three different accessible phases on the Earth’s surface: vapor, liquid, and ice. In fact, water has many high-pressure ice phases, which are all unique in their crystal structure. This means that the way in which the atoms are bonded together into a solid network differ between different solid ...
... has three different accessible phases on the Earth’s surface: vapor, liquid, and ice. In fact, water has many high-pressure ice phases, which are all unique in their crystal structure. This means that the way in which the atoms are bonded together into a solid network differ between different solid ...
PCSD General Chemistry Pacing Guide
... Objectives Describe the types of radioactive decay Write nuclear equations that describe radioactive ...
... Objectives Describe the types of radioactive decay Write nuclear equations that describe radioactive ...
One-Particle Density Matrix Functional for Correlation in Molecular
... that the 2-RDM corresponds to an N-particle wave function is not known (the N-representability problem) [3]. The minimization of the energy with respect to a 2-RDM constrained by the well-known D, Q, and G necessary conditions [3, 4] has been recently presented [5] with accurate results. The contrac ...
... that the 2-RDM corresponds to an N-particle wave function is not known (the N-representability problem) [3]. The minimization of the energy with respect to a 2-RDM constrained by the well-known D, Q, and G necessary conditions [3, 4] has been recently presented [5] with accurate results. The contrac ...
Unit Powerpoint
... In the Table of Standard Reduction Potentials that zinc has a negative E° indicating that it is not as good at competing for electrons as hydrogen. Zn2+(aq) + 2e- → Zn(s) E° = -0.76 V Therefore if zinc and hydrogen are paired together in an electrochemical cell, the hydrogen would be reduced (gain t ...
... In the Table of Standard Reduction Potentials that zinc has a negative E° indicating that it is not as good at competing for electrons as hydrogen. Zn2+(aq) + 2e- → Zn(s) E° = -0.76 V Therefore if zinc and hydrogen are paired together in an electrochemical cell, the hydrogen would be reduced (gain t ...
Louis de Broglie - Nobel Lecture
... of reality, it must be possible to establish a certain parallelism between the motion of a corpuscle and the propagation of the associated wave. The first objective to achieve had, therefore, to be to establish this correspondence. With that in view I started by considering the simplest case: that o ...
... of reality, it must be possible to establish a certain parallelism between the motion of a corpuscle and the propagation of the associated wave. The first objective to achieve had, therefore, to be to establish this correspondence. With that in view I started by considering the simplest case: that o ...
Remarks on energetic conditions for positronium formation in non
... molecule electrons. Furthermore, some other features such as the timescale of relaxation, due to a positron annihilation, cannot be transferred. These difficulties cause that there is not present in the literature a quantum model of positron interaction with condensed matter. Furthermore, since the pr ...
... molecule electrons. Furthermore, some other features such as the timescale of relaxation, due to a positron annihilation, cannot be transferred. These difficulties cause that there is not present in the literature a quantum model of positron interaction with condensed matter. Furthermore, since the pr ...
Classical support for non-dispersive two
... The most prominent of those has probably been the discovery of a new, highly correlated and globally stable electronic configuration, the ‘frozen planet’ [1, 2]. The appeal of this configuration resides in its counterintuitive, asymmetric character where both electrons are located on the same side o ...
... The most prominent of those has probably been the discovery of a new, highly correlated and globally stable electronic configuration, the ‘frozen planet’ [1, 2]. The appeal of this configuration resides in its counterintuitive, asymmetric character where both electrons are located on the same side o ...
CHAPTER 2
... • -Sir John Joseph Thompson and Ernest Rutherford established a model of the atom still in use today • -Three fundamental particles make-up atoms: Particle ...
... • -Sir John Joseph Thompson and Ernest Rutherford established a model of the atom still in use today • -Three fundamental particles make-up atoms: Particle ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.