Measuring Fractional Quantum Hall Effect
... electrons on the one side of the sample is big enough for Ey to cancel out the Lorentz force due to the magnetic field, the electrons will again on average move in the longitudinal direction[2]. From the Hall voltage one can calculate the Hall resistance[1]: ...
... electrons on the one side of the sample is big enough for Ey to cancel out the Lorentz force due to the magnetic field, the electrons will again on average move in the longitudinal direction[2]. From the Hall voltage one can calculate the Hall resistance[1]: ...
Image Potential and Charge-Transfer Phenomena in Atom (Ion
... There have been many quantum-mechanical, or semi-classica16, calculations of the image potential for a charged particle near a metallic surface3p7-12. In most approaches the image potential is obtained by calculating the self-energy of the charged particle in the presence of a surface, which usually ...
... There have been many quantum-mechanical, or semi-classica16, calculations of the image potential for a charged particle near a metallic surface3p7-12. In most approaches the image potential is obtained by calculating the self-energy of the charged particle in the presence of a surface, which usually ...
Atomistic description of wave function localization effects in InxGa1
... 3.2 Electronic structure of Inx Ga1−x N/GaN QWs In this section we analyze the electronic structure of In0.25 Ga0.75 N/GaN QWs by means of our TB model. As a model system we assume a 3.5 nm thick In0.25 Ga0.75 N/GaN QW. All QW calculations have been performed on SCs containing ≈ 82,000 atoms (≈ 10nm ...
... 3.2 Electronic structure of Inx Ga1−x N/GaN QWs In this section we analyze the electronic structure of In0.25 Ga0.75 N/GaN QWs by means of our TB model. As a model system we assume a 3.5 nm thick In0.25 Ga0.75 N/GaN QW. All QW calculations have been performed on SCs containing ≈ 82,000 atoms (≈ 10nm ...
Momentum Transfer to a Free Floating Double Slit
... Quantum mechanics poses a major challenge to our intuition which is trained in the macrocosm to the laws of classical physics. Among all the quantum phenomena, the double-slit interference is ‘‘a phenomenon which is impossible (. . .) to explain in any classical way, and which has in it the heart of ...
... Quantum mechanics poses a major challenge to our intuition which is trained in the macrocosm to the laws of classical physics. Among all the quantum phenomena, the double-slit interference is ‘‘a phenomenon which is impossible (. . .) to explain in any classical way, and which has in it the heart of ...
Modern Methods in Drug Discovery
... The problem of ab initio calculation is their N4 dependence from the number of two-electron integrals. These arise from the number of basis functions and the interactions between electrons on different atoms. In semiempirical methods the numerical effort is strongly reduced by assumptions and approa ...
... The problem of ab initio calculation is their N4 dependence from the number of two-electron integrals. These arise from the number of basis functions and the interactions between electrons on different atoms. In semiempirical methods the numerical effort is strongly reduced by assumptions and approa ...
Lec. 42 notes
... Each atomic electron can be identified by four quantum numbers: n = 1, 2, 3,… = principal quantum number ℓ gives total orbital angular momentum: m gives z-component of orbital angular momentum: ms = ±½ gives the z-component of spin: The atom itself has angular momentum which is the vector sum of orb ...
... Each atomic electron can be identified by four quantum numbers: n = 1, 2, 3,… = principal quantum number ℓ gives total orbital angular momentum: m gives z-component of orbital angular momentum: ms = ±½ gives the z-component of spin: The atom itself has angular momentum which is the vector sum of orb ...
Document
... Why does the Stern‐Gerlach experiment show that the operator “ measure the z component of the magnetic moment of an Ag atom” has only two eigenfunctions with eigenvalues that have the same magnitude and opposite sign? Q17.2 Have a closer look at Equation (17.6) and Figure 17.4. How would Figure ...
... Why does the Stern‐Gerlach experiment show that the operator “ measure the z component of the magnetic moment of an Ag atom” has only two eigenfunctions with eigenvalues that have the same magnitude and opposite sign? Q17.2 Have a closer look at Equation (17.6) and Figure 17.4. How would Figure ...
UNIT 2 ATOMS, MATTER, AND THE MOLE
... there are two atoms of hydrogen. 2. H2O2 is not water. It is called hydrogen peroxide, has two atoms of hydrogen for every two atoms of oxygen and behaves much differently that water. This brings us to the next law. F. LAW OF MULTIPLE PROPORTIONS-states that there can exist two or more compounds wit ...
... there are two atoms of hydrogen. 2. H2O2 is not water. It is called hydrogen peroxide, has two atoms of hydrogen for every two atoms of oxygen and behaves much differently that water. This brings us to the next law. F. LAW OF MULTIPLE PROPORTIONS-states that there can exist two or more compounds wit ...
Chemistry General v. 2016
... Dalton’s Atomic Theory- the belief that all matter is composed of tiny, indivisible elements; IsotopeAn atom that has the same number of protons as other atoms of the same element do but that has different number of neutrons; Law of Conservation of Mass- Mass cannot be created or destroyed in ordina ...
... Dalton’s Atomic Theory- the belief that all matter is composed of tiny, indivisible elements; IsotopeAn atom that has the same number of protons as other atoms of the same element do but that has different number of neutrons; Law of Conservation of Mass- Mass cannot be created or destroyed in ordina ...
Atomic Physics - NMSU Astronomy
... The model of the atom is simple in principle, yet highly complex in detail. Employing only first–order physics, we have been extremely successful at formulating the basic atomic model, including the internal energy structure, transition probabilities, and the spectra of the various atoms and ions. H ...
... The model of the atom is simple in principle, yet highly complex in detail. Employing only first–order physics, we have been extremely successful at formulating the basic atomic model, including the internal energy structure, transition probabilities, and the spectra of the various atoms and ions. H ...
Coulombic interactions in the fractional quantum Hall effect: from
... where rc2 := (x − X0 )2 + (y − Y0 )2 . Now, the angular momentum is indeed a constant of motion, since [H, L] = 0. But the physical meaning of L in the presence of an external magnetic field can be very unusual: in this context it quantifies the radial position of the orbit center. Let us write the ...
... where rc2 := (x − X0 )2 + (y − Y0 )2 . Now, the angular momentum is indeed a constant of motion, since [H, L] = 0. But the physical meaning of L in the presence of an external magnetic field can be very unusual: in this context it quantifies the radial position of the orbit center. Let us write the ...
Lanthanides and Actinides
... actinides, which cause a huge number of spectral lines. A simple model is needed to extract the basic features of the electronic structure and to enable a successful analysis of the experimentally determined data to be made. For an experimental chemist the exact quantum mechanical classification of ...
... actinides, which cause a huge number of spectral lines. A simple model is needed to extract the basic features of the electronic structure and to enable a successful analysis of the experimentally determined data to be made. For an experimental chemist the exact quantum mechanical classification of ...
Quantum Entanglements and Hauntological Relations of Inheritance
... A closer examination brings the spectral quality of this process to light. Initially, the electron is in some higher energy state E2 , and then in some lower energy state E1 . At what point is the photon emitted? On Rutherford’s classical physics account, an atomic electron can have a continuous ran ...
... A closer examination brings the spectral quality of this process to light. Initially, the electron is in some higher energy state E2 , and then in some lower energy state E1 . At what point is the photon emitted? On Rutherford’s classical physics account, an atomic electron can have a continuous ran ...
Pauli Exclusion Principle
... 7-74 If relativistic effects are ignored, the n = 3 level for one-electron atoms consists of the 32S1/2, 32P1/2, 32P3/2, 32D3/2, and 32D5/2 states. Compute the spin-orbit-effect splittings of 3P and 3D states for hydrogen. ...
... 7-74 If relativistic effects are ignored, the n = 3 level for one-electron atoms consists of the 32S1/2, 32P1/2, 32P3/2, 32D3/2, and 32D5/2 states. Compute the spin-orbit-effect splittings of 3P and 3D states for hydrogen. ...
Atomic Physics
... combinations of ml and ms .) Notation for the ground state of an atom: n lnumber of electrons . Hydrogen(H): 1 electron,1 s1 . Helium (He): 2 electrons, 1 s2 . Lithium (Li): 3 electrons, 1 s2 2 s1 . Beryllium (Be): 4 electrons, 1 s2 2 s2 ...
... combinations of ml and ms .) Notation for the ground state of an atom: n lnumber of electrons . Hydrogen(H): 1 electron,1 s1 . Helium (He): 2 electrons, 1 s2 . Lithium (Li): 3 electrons, 1 s2 2 s1 . Beryllium (Be): 4 electrons, 1 s2 2 s2 ...
Atomic orbital
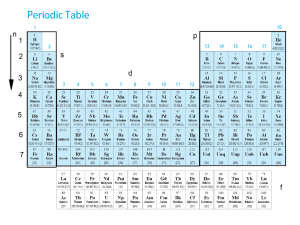
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.