Optical probing of the spin state of a single magnetic impurity in a
... + cmh共2x + 2y 兲 − Ee−h / 2. Since the coefficients ⌬z0,1 are typically negative, the ground states are ⌽±1,⫿1/2 and correspond to 兩Jz 兩 = 1 and Jtot,z = Jz + sz = ± 1 / 2 [see Fig. 2(b)]. We now calculate the interband optical matrix elements responsible for the photoluminescence (PL) process, ass ...
... + cmh共2x + 2y 兲 − Ee−h / 2. Since the coefficients ⌬z0,1 are typically negative, the ground states are ⌽±1,⫿1/2 and correspond to 兩Jz 兩 = 1 and Jtot,z = Jz + sz = ± 1 / 2 [see Fig. 2(b)]. We now calculate the interband optical matrix elements responsible for the photoluminescence (PL) process, ass ...
chapter-26
... (Planck’s Constant, a Proportionality Constant) 6.626 x 10-34 Js) 6.626 x 10-34 kgm2/s – Atoms, therefore, emit only certain quantities of energy and the energy of an atom is described as being “quantized” – Thus, an atom changes its energy state by emitting (or absorbing) one or more quanta T.Nor ...
... (Planck’s Constant, a Proportionality Constant) 6.626 x 10-34 Js) 6.626 x 10-34 kgm2/s – Atoms, therefore, emit only certain quantities of energy and the energy of an atom is described as being “quantized” – Thus, an atom changes its energy state by emitting (or absorbing) one or more quanta T.Nor ...
Chemistry Packet: Chemical Bonding
... can be anywhere in the range between these two extremes, depending upon how strongly the bonded atoms attract electrons. ...
... can be anywhere in the range between these two extremes, depending upon how strongly the bonded atoms attract electrons. ...
CH1 Student Revision Guides pdf
... It is known that exposure to radiation can cause cell mutation leading to carcinomas and to forms of leukaemia. Even small increases in the background level of radiation may have significant effects on the population as a whole. This is because the probability for cell mutation is higher when appli ...
... It is known that exposure to radiation can cause cell mutation leading to carcinomas and to forms of leukaemia. Even small increases in the background level of radiation may have significant effects on the population as a whole. This is because the probability for cell mutation is higher when appli ...
Chemistry Review Module Chapter 1
... representation of the valence electrons of an atom – When sketching an atom, write the symbol, and then arrange dots around it to represent its valence electrons. 2 paired electrons – Example: N has 5 valence electrons N 3 “odd” unpaired electrons – The “odd” or unpaired electrons are available fo ...
... representation of the valence electrons of an atom – When sketching an atom, write the symbol, and then arrange dots around it to represent its valence electrons. 2 paired electrons – Example: N has 5 valence electrons N 3 “odd” unpaired electrons – The “odd” or unpaired electrons are available fo ...
On The Copenhagen Interpretation of Quantum Mechanics
... mechanics were mathematically equivalent. That is, they were just different mathematical ways of expressing the same underlying structure. Please do not ask me to explain here how a theory for a thing that waves can be equivalent to a theory based on infinite arrays of numbers. Mathematically it is ...
... mechanics were mathematically equivalent. That is, they were just different mathematical ways of expressing the same underlying structure. Please do not ask me to explain here how a theory for a thing that waves can be equivalent to a theory based on infinite arrays of numbers. Mathematically it is ...
Atom cooling, trapping, and quantum manipulation
... twice the photon momentum. When the atom waves travel through a thick standing wave, one diffracted order predominates. For a thin standing wave, the diffraction pattern is spread over many orders. Blazed gratings have also been demonstrated, as well as Raman and adiabatic-dark-state gratings in whi ...
... twice the photon momentum. When the atom waves travel through a thick standing wave, one diffracted order predominates. For a thin standing wave, the diffraction pattern is spread over many orders. Blazed gratings have also been demonstrated, as well as Raman and adiabatic-dark-state gratings in whi ...
Multivalent Ionic Compounds
... Columns of elements are called o All elements in a family have similar properties, and bond with other elements in similar ways ...
... Columns of elements are called o All elements in a family have similar properties, and bond with other elements in similar ways ...
Phys. Rev. Lett. 93, 073002
... and beam waists (1=e2 ) of w # 150 "m at the position of the BEC. We convert the BEC into a Mott insulator by gradually increasing the potential depth of the 3D lattice to a value of up to 27Er over a time of 80 ms. Here Er # h! 2 k2 =2m denotes the recoil energy, m the mass of a rubidium atom, and ...
... and beam waists (1=e2 ) of w # 150 "m at the position of the BEC. We convert the BEC into a Mott insulator by gradually increasing the potential depth of the 3D lattice to a value of up to 27Er over a time of 80 ms. Here Er # h! 2 k2 =2m denotes the recoil energy, m the mass of a rubidium atom, and ...
Lecture 03B - Balancing Redox
... Rule 3: Atoms in polyatomic ions or molecular compounds usually have O.N.s identical to the charges they would have as ions. Exceptions: -Hydrogen (except as H2) is usually +1, but sometimes -1 (ex. H-Ca-H) -Oxygen (except as O2) is usually -2, but can be -1 when –O-O- bonds exist (peroxides) -Halog ...
... Rule 3: Atoms in polyatomic ions or molecular compounds usually have O.N.s identical to the charges they would have as ions. Exceptions: -Hydrogen (except as H2) is usually +1, but sometimes -1 (ex. H-Ca-H) -Oxygen (except as O2) is usually -2, but can be -1 when –O-O- bonds exist (peroxides) -Halog ...
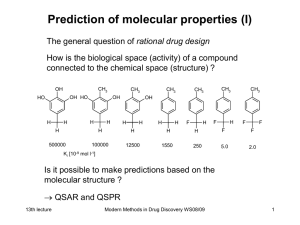
Modern Methods in Drug Discovery
... Besides the structure of molecules all other electronic properties can be calculated. Many of those result as response of the molecule to an external disturbance: Removal of one electron ionization potential In general a disturbance by an electric field can be expressed in the form of a Taylor exp ...
... Besides the structure of molecules all other electronic properties can be calculated. Many of those result as response of the molecule to an external disturbance: Removal of one electron ionization potential In general a disturbance by an electric field can be expressed in the form of a Taylor exp ...
IOSR Journal of Applied Physics (IOSR-JAP)
... the shapeare the same except that, in case of muon the distributions are more closely to the proton center, and the overlap with the potential is more than that of the electron,which explains the higher values of the vacuum polarization corrections in the energy levels in case of muon than in case o ...
... the shapeare the same except that, in case of muon the distributions are more closely to the proton center, and the overlap with the potential is more than that of the electron,which explains the higher values of the vacuum polarization corrections in the energy levels in case of muon than in case o ...
Super-charging nonlinear optical processes through collective effects
... Decoherence Mechanisms Atomic Motion • Expect gratings to decay due to ballistic atomic motion in a time τc ...
... Decoherence Mechanisms Atomic Motion • Expect gratings to decay due to ballistic atomic motion in a time τc ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.