History of Atomic Theory PowerPoint
... atoms were made up of smaller parts through his experiments with a cathode ray ...
... atoms were made up of smaller parts through his experiments with a cathode ray ...
From atoms to the periodic table
... planar gap) at the nucleus. These orbitals are known as p-‐orbitals and they come in sets of three, oriented at 90° to each other. The colours here represent different phases. Other nota9ons, in ...
... planar gap) at the nucleus. These orbitals are known as p-‐orbitals and they come in sets of three, oriented at 90° to each other. The colours here represent different phases. Other nota9ons, in ...
QTMN-16.107-166, Layout 1
... for the outer electrons, where σ is sc. nuclear screening constant. This is also called as shielding. However, the outer electron orbital may penetrate through the inner core orbitals close to the nucleus making the shape of the orbitals and distance from the nucleus essential factors in shielding. ...
... for the outer electrons, where σ is sc. nuclear screening constant. This is also called as shielding. However, the outer electron orbital may penetrate through the inner core orbitals close to the nucleus making the shape of the orbitals and distance from the nucleus essential factors in shielding. ...
Alkali Elements Alkali Elements: Excited States
... Most of the energetics of these atoms is well described by the Hartree model; however, in detail (e.g. in high-resolution spectroscopy), spin-orbit coupling and the residual coulomb interaction are important. Residual Coulomb Interaction: The Coulomb interaction that is not captured by the effective ...
... Most of the energetics of these atoms is well described by the Hartree model; however, in detail (e.g. in high-resolution spectroscopy), spin-orbit coupling and the residual coulomb interaction are important. Residual Coulomb Interaction: The Coulomb interaction that is not captured by the effective ...
Chapter 8: Periodic Properties of the Elements
... elements in a group, you can make a good guess at the properties of the other elements in the same group. Periods are the horizontal rows in the periodic table. Many patterns can be seen or predicted following periods and groups. Electron Configurations and Orbital Diagrams: ...
... elements in a group, you can make a good guess at the properties of the other elements in the same group. Periods are the horizontal rows in the periodic table. Many patterns can be seen or predicted following periods and groups. Electron Configurations and Orbital Diagrams: ...
Lecture 1.1 Some preliminary chemistry knowledge, ppt file
... Refresh your high school chemistry ...
... Refresh your high school chemistry ...
Environment Assisted Quantum Transport in Organic Molecules
... where Lrs is a positive definite covariance matrix of the noise at different atomic sites. We can get Lrs from detailed models. The crudest approximation is when we assume a completely uncorrelated external noise and set Lrs = Γh̄ δrs , where Γ is the strength of the decoherence. Its detailed form i ...
... where Lrs is a positive definite covariance matrix of the noise at different atomic sites. We can get Lrs from detailed models. The crudest approximation is when we assume a completely uncorrelated external noise and set Lrs = Γh̄ δrs , where Γ is the strength of the decoherence. Its detailed form i ...
WS on obj. 1-11
... 3. _____ (T/F) The number of valence electrons is very important in determining the chemical properties of an element. 4. _____ (T/F) The elements of a group have different numbers of valence electrons. 5. _____ (T/F) The representative groups 1A-7A have the same number of valence electrons as their ...
... 3. _____ (T/F) The number of valence electrons is very important in determining the chemical properties of an element. 4. _____ (T/F) The elements of a group have different numbers of valence electrons. 5. _____ (T/F) The representative groups 1A-7A have the same number of valence electrons as their ...
Lecture28
... • Yet another quantum number was introduced when it was discovered that the spectral lines of a gas are actually split into two closely spaced lines (fine structure) even without a strong magnetic field due to spinning of electrons. Spin magnetic quantum number ms : ms =-1/2,+1/2 Number of allowed ...
... • Yet another quantum number was introduced when it was discovered that the spectral lines of a gas are actually split into two closely spaced lines (fine structure) even without a strong magnetic field due to spinning of electrons. Spin magnetic quantum number ms : ms =-1/2,+1/2 Number of allowed ...
Chapter 2 – The Structure of the Atom Since the book assumes you
... b) The total distance of the box is fixed. Conceptually, however, the problems are the same and, as a result, their solutions are related. The Hydrogen Atom Solving the Schrödinger equation for the hydrogen atom is very similar to doing so for the particle-in-a-box. In addition to the 3 requirements ...
... b) The total distance of the box is fixed. Conceptually, however, the problems are the same and, as a result, their solutions are related. The Hydrogen Atom Solving the Schrödinger equation for the hydrogen atom is very similar to doing so for the particle-in-a-box. In addition to the 3 requirements ...
Document
... in discrete packets, or quanta. The energy of these quanta is proportional to the frequency of the radiation. ...
... in discrete packets, or quanta. The energy of these quanta is proportional to the frequency of the radiation. ...
3.5 Why does a quantum mechanic state change?
... For all these processes according to Eq. (3.18) the transfer matrix element can be calculated; they quantize the probability for these transitions. The excited state may loose energy by the same processes as described above: • by electromagnetic radiation • the same particles, which moved into the s ...
... For all these processes according to Eq. (3.18) the transfer matrix element can be calculated; they quantize the probability for these transitions. The excited state may loose energy by the same processes as described above: • by electromagnetic radiation • the same particles, which moved into the s ...
An Overview of Computational Chemistry
... Limitations of the Born-Oppenheimer approximation •The total wave function is limited to one electronic surface, i.e. a particular electronic state. •The BO approx. is usually very good, but breaks down when two (or more) electronic states are close in energy at particular nuclear geometries. •In su ...
... Limitations of the Born-Oppenheimer approximation •The total wave function is limited to one electronic surface, i.e. a particular electronic state. •The BO approx. is usually very good, but breaks down when two (or more) electronic states are close in energy at particular nuclear geometries. •In su ...
Energy levels and atomic structures lectures
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on ...
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on ...
Atomic Structure
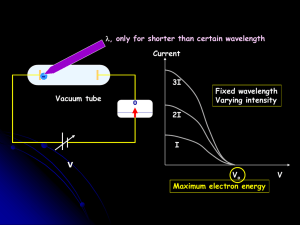
... Classical physics, when applied to black body radiation, predicted that the intensity of the radiation emitted would dramatically increase at shorter and shorter wavelengths. The result was that any hot body should emit intense UV radiation, and even x-rays. Even a human body at 37oC would glow in t ...
... Classical physics, when applied to black body radiation, predicted that the intensity of the radiation emitted would dramatically increase at shorter and shorter wavelengths. The result was that any hot body should emit intense UV radiation, and even x-rays. Even a human body at 37oC would glow in t ...
do with electron orbitals?
... I. The energy of the ground state solution is ________ II. The angular momentum of the ground state solution is different _______ different III. The location of the electron is _______ a. same, same, same b. same, same, different c. same, different, different d. different, same, different e. differe ...
... I. The energy of the ground state solution is ________ II. The angular momentum of the ground state solution is different _______ different III. The location of the electron is _______ a. same, same, same b. same, same, different c. same, different, different d. different, same, different e. differe ...
Lectures 3-4: Quantum mechanics of one
... o We have now separated the time-independent Schrödinger equation into three differential equations, which each only depend on one of # (4), $ (5) and R(6). ...
... o We have now separated the time-independent Schrödinger equation into three differential equations, which each only depend on one of # (4), $ (5) and R(6). ...
Models of an atom and old quantum theory
... electromagnetic waves because their motion is accelerated. In this manner they must loose energy and eventually slow down and fall on the nucleus (in about 10−12 s). A stable atom would be rather similar to the Thompson's atom, but of much smaller size (∼ 10−14 m) than observed. The resolution of th ...
... electromagnetic waves because their motion is accelerated. In this manner they must loose energy and eventually slow down and fall on the nucleus (in about 10−12 s). A stable atom would be rather similar to the Thompson's atom, but of much smaller size (∼ 10−14 m) than observed. The resolution of th ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.