(normal) Zeeman Effect with Spin Spin
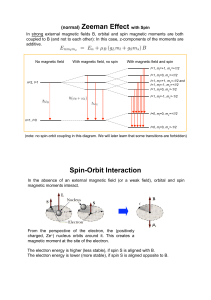
... 2s+1 : “Spin Multiplicity” " total number of spin states [e.g. 2s+1=2 (up and down) for s=1/2]. ...
... 2s+1 : “Spin Multiplicity” " total number of spin states [e.g. 2s+1=2 (up and down) for s=1/2]. ...
n - WordPress.com
... • Rydberg analyzed the spectrum of hydrogen and found that it could be described with an equation that involved an inverse square of integers ...
... • Rydberg analyzed the spectrum of hydrogen and found that it could be described with an equation that involved an inverse square of integers ...
64-311/5: Atomic and Molecular Spectra
... The Schrodinger model for hydrogen agreed with the Bohr model in that they both resulted in the energies of the excited states being only a function of principal quantum number n. This 'accidental degeneracy' for the different l states is not strictly true as closer scrutiny of the spectral lines re ...
... The Schrodinger model for hydrogen agreed with the Bohr model in that they both resulted in the energies of the excited states being only a function of principal quantum number n. This 'accidental degeneracy' for the different l states is not strictly true as closer scrutiny of the spectral lines re ...
I. Waves & Particles
... shines on the metal Hmm… (For a given metal, no electrons were emitted if the light’s frequency was below a certain minimum – why did light have to be of a minimum frequency?) ...
... shines on the metal Hmm… (For a given metal, no electrons were emitted if the light’s frequency was below a certain minimum – why did light have to be of a minimum frequency?) ...
The Periodic Table
... Reason: electrons added in the same principal quantum level do not completely shield the increasing nuclear charge caused by the added protons. The electrons in the same principal quantum level are generally more strongly bound when moving left to right across the periodic table (NOTE: This trend is ...
... Reason: electrons added in the same principal quantum level do not completely shield the increasing nuclear charge caused by the added protons. The electrons in the same principal quantum level are generally more strongly bound when moving left to right across the periodic table (NOTE: This trend is ...
QM_2_particles_ver2
... a) Schrodinger writes a generalized equation that deBroglie waves must obey when there is Potential Energy (such that the wavelength changes from point to point in space) ...
... a) Schrodinger writes a generalized equation that deBroglie waves must obey when there is Potential Energy (such that the wavelength changes from point to point in space) ...
atoms
... The answer is D. Atoms are the basic building blocks of all matter and have the same general structure, including a nucleus and electrons. Elements found in both living and nonliving things are made of atoms. ...
... The answer is D. Atoms are the basic building blocks of all matter and have the same general structure, including a nucleus and electrons. Elements found in both living and nonliving things are made of atoms. ...
Chapter 3 Atomic Structure
... When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns to a lower energy state, it emits a ph ...
... When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns to a lower energy state, it emits a ph ...
15. Crafting the Quantum.IV
... • Electron states in an atom are uniquely characterized by 4 quantum numbers: principle n, azimuthal k, and two magnetic numbers m1, m2. • These states obey an "Exclusion Principle": "There can never be two or more equivalent electrons in an atom for which, in strong fields, the values of all quantu ...
... • Electron states in an atom are uniquely characterized by 4 quantum numbers: principle n, azimuthal k, and two magnetic numbers m1, m2. • These states obey an "Exclusion Principle": "There can never be two or more equivalent electrons in an atom for which, in strong fields, the values of all quantu ...
Chemistry Lesson Plans #12
... radiant energy (E) absorbed or emitted by a body is proportional to the frequency of the radiation • E ∝ v or E = hiv • h is another fudge factor called Planck’s constant and has a value of 6.6262x10-34J·s, where J is joule, the SI unit for energy. • The energy of a quantum then is hiv , and any att ...
... radiant energy (E) absorbed or emitted by a body is proportional to the frequency of the radiation • E ∝ v or E = hiv • h is another fudge factor called Planck’s constant and has a value of 6.6262x10-34J·s, where J is joule, the SI unit for energy. • The energy of a quantum then is hiv , and any att ...
End Show
... What does the quantum mechanical model determine about the electrons in an atom? The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations Slide around the nucleus. 6 of 26 © Copyright Pearson Prentice Hall ...
... What does the quantum mechanical model determine about the electrons in an atom? The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations Slide around the nucleus. 6 of 26 © Copyright Pearson Prentice Hall ...
10mod_phys
... problems with the Rutherford model. Proposed: – An electron can only occupy certain allowed orbits without radiating – Each nth orbit has a radius (rn) and an energy (En). – An electron can make a transition between two orbits through • Absorbing a Photon (ELOWER EHIGHER) • Emitting a Photon (EHIG ...
... problems with the Rutherford model. Proposed: – An electron can only occupy certain allowed orbits without radiating – Each nth orbit has a radius (rn) and an energy (En). – An electron can make a transition between two orbits through • Absorbing a Photon (ELOWER EHIGHER) • Emitting a Photon (EHIG ...
The de Broglie-Bohr Model for the Hydrogen Atom
... 1926. However, Bohr's model is still profitably taught today because of its conceptual and mathematical simplicity, and because it introduced a number of key quantum mechanical ideas such as the quantum number, quantization of observable properties, quantum jump and stationary state. Bohr calculated ...
... 1926. However, Bohr's model is still profitably taught today because of its conceptual and mathematical simplicity, and because it introduced a number of key quantum mechanical ideas such as the quantum number, quantization of observable properties, quantum jump and stationary state. Bohr calculated ...
The Electronic Structures of Atoms Electromagnetic Radiation The
... Energy is absorbed when electrons jump to higher orbits. n = 2 to n = 4 for example Energy is emitted when electrons fall to lower orbits. n = 4 to n = 1 for example ...
... Energy is absorbed when electrons jump to higher orbits. n = 2 to n = 4 for example Energy is emitted when electrons fall to lower orbits. n = 4 to n = 1 for example ...
electrons - TAMU Chemistry
... The relationship between wavelength and frequency for any wave is velocity = . For electromagnetic radiation the velocity is 3.00 x 108 m/s and has the symbol c. Thus c = for electromagnetic radiation. ...
... The relationship between wavelength and frequency for any wave is velocity = . For electromagnetic radiation the velocity is 3.00 x 108 m/s and has the symbol c. Thus c = for electromagnetic radiation. ...
Lecture 21 revised (Slides) October 12
... we’d see that for each value of n there is one s orbital (unique ml value), for n ≥ 2 three p orbitals (three ml values) and, for n ≥ 3, five d orbitals (five ml values: -2, -1, 0, +1, +2). This means that s, p, d subshells can contain (at most) 2, 6 and 10 electrons respectively. Relative subshell ...
... we’d see that for each value of n there is one s orbital (unique ml value), for n ≥ 2 three p orbitals (three ml values) and, for n ≥ 3, five d orbitals (five ml values: -2, -1, 0, +1, +2). This means that s, p, d subshells can contain (at most) 2, 6 and 10 electrons respectively. Relative subshell ...
Chapter 2 - Molecular orbital theory
... De Broglie, 1924: Wave-particle duality describes electromagnetic radiation and matter (such as electrons, protons...) Schrodinger equation: Accounts for wave-particle duality, the motion of electrons in an atom, and quantized nature of atomic structure ...
... De Broglie, 1924: Wave-particle duality describes electromagnetic radiation and matter (such as electrons, protons...) Schrodinger equation: Accounts for wave-particle duality, the motion of electrons in an atom, and quantized nature of atomic structure ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.