Spin Polarized Electron - Jordan University of Science and
... polarized electron :If the electron spins have a preferential orientation so that there exists a direction for which the two possible spin states are not equally populated . Polarization is defined :- ...
... polarized electron :If the electron spins have a preferential orientation so that there exists a direction for which the two possible spin states are not equally populated . Polarization is defined :- ...
No Slide Title
... The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. ...
... The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. ...
Assignment Chemistry Class XI (2016-17)
... 12. Magnitude of force F experienced by a certain object moving with speed v is given by F = Kv2 where K is a constant. Find the dimensions of K. 13. The length and breadth of a rectangle are measured as (a± Δa) and (b±Δb) respectively. Find (i) relative error , (ii) absolute error in the measuremen ...
... 12. Magnitude of force F experienced by a certain object moving with speed v is given by F = Kv2 where K is a constant. Find the dimensions of K. 13. The length and breadth of a rectangle are measured as (a± Δa) and (b±Δb) respectively. Find (i) relative error , (ii) absolute error in the measuremen ...
III. Quantum Model of the Atom
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
III. Quantum Model of the Atom
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
Chapter 4 4.1 Defining the Atom • Early Models of the Atom atom
... one element are different from those of any other element 3) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4) Chemical reactions occur when atoms are separated from each other, joined, or rearranged in a different c ...
... one element are different from those of any other element 3) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4) Chemical reactions occur when atoms are separated from each other, joined, or rearranged in a different c ...
III. Quantum Model of the Atom
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
Problem Set 11
... (c) What is the penetration length δ for the wave function in (b)? (Hint: the penetration length is the length at which the wave function is equal to 1/e.) (d) Compute the penetration length δ, using E = 1 eV, V0 = 1.1 eV, a = 0.1 nm, and me = 511 keV. (e) The transmission coefficient in this region ...
... (c) What is the penetration length δ for the wave function in (b)? (Hint: the penetration length is the length at which the wave function is equal to 1/e.) (d) Compute the penetration length δ, using E = 1 eV, V0 = 1.1 eV, a = 0.1 nm, and me = 511 keV. (e) The transmission coefficient in this region ...
Glossary
... Empirical formula − a molecular formula with smallest integer ratio subscripts. Energy − a measure of the actual (kinetic) or potential (work) motion of a system. Energy level − descriptive term for quantized energy states in atoms and molecules, derived from visualizing discontinuous horizontal lin ...
... Empirical formula − a molecular formula with smallest integer ratio subscripts. Energy − a measure of the actual (kinetic) or potential (work) motion of a system. Energy level − descriptive term for quantized energy states in atoms and molecules, derived from visualizing discontinuous horizontal lin ...
Lecture 5 - Help-A-Bull
... Relate the radius of an atom to an ion of the same element Describe the trends in ionization energy on the periodic table and relate the observed trends to the structure of the atom Predict the expected trends in successive ionization energies Define electron affinity Describe what is meant by metal ...
... Relate the radius of an atom to an ion of the same element Describe the trends in ionization energy on the periodic table and relate the observed trends to the structure of the atom Predict the expected trends in successive ionization energies Define electron affinity Describe what is meant by metal ...
The Quantum Mechanical Model of the Atom
... atoms. Therefore, a simple modification of Bohr’s atomic model was not Each of these sub-levels itsearly own energy Byhas the 1920s, it was standard knowledge that energy had matter-like enough. The many-electron problem called for a new model to explain properties. In 1924, a young physics student ...
... atoms. Therefore, a simple modification of Bohr’s atomic model was not Each of these sub-levels itsearly own energy Byhas the 1920s, it was standard knowledge that energy had matter-like enough. The many-electron problem called for a new model to explain properties. In 1924, a young physics student ...
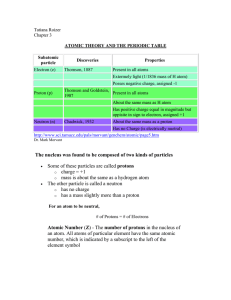
atomic theory and the periodic table
... Properties of the elements recur in regular cycles (periodically) when the elements are arranged in order of increasing atomic weight. ...
... Properties of the elements recur in regular cycles (periodically) when the elements are arranged in order of increasing atomic weight. ...
BatelaanUpdate
... field lines. Is this evidence for electromagnetic forces that work in new and unsuspected ways? Or is it just that infamous source of Albert Einstein's discomfort - quantummechanical 'spooky action at a distance'? In the latest chapter in an involved history, detailed in Physical Review Letters, Ca ...
... field lines. Is this evidence for electromagnetic forces that work in new and unsuspected ways? Or is it just that infamous source of Albert Einstein's discomfort - quantummechanical 'spooky action at a distance'? In the latest chapter in an involved history, detailed in Physical Review Letters, Ca ...
Chapter 2 - Las Positas College
... Q29.23. Reason: The electron gives up some of its energy to the atom. At atom in its ground state cannot emit a photon, so the atom is first boosted to an excited state (one of the orbital electrons jumps to a higher state) and then it can emit a photon as it drops to a lower state. If the excited e ...
... Q29.23. Reason: The electron gives up some of its energy to the atom. At atom in its ground state cannot emit a photon, so the atom is first boosted to an excited state (one of the orbital electrons jumps to a higher state) and then it can emit a photon as it drops to a lower state. If the excited e ...
chapter02_part1_lecture - bloodhounds Incorporated
... number of protons (positively charged) is equal to the number of electrons ...
... number of protons (positively charged) is equal to the number of electrons ...
ψ 2
... molecular charge distribution) for H2 in a plane containing the nuclei. Also shown is a profile of the density distribution along the internuclear axis. The internuclear separation is 1.4 au. The values of the contours increase in magnitude from the outermost one inwards towards the nuclei. The valu ...
... molecular charge distribution) for H2 in a plane containing the nuclei. Also shown is a profile of the density distribution along the internuclear axis. The internuclear separation is 1.4 au. The values of the contours increase in magnitude from the outermost one inwards towards the nuclei. The valu ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.