Metal d orbitals in an O crystal field
... In the crystal field theory (CFT) model, the spectrochemical series is an empirical result that cannot be rationalized in terms of simple point charges. ...
... In the crystal field theory (CFT) model, the spectrochemical series is an empirical result that cannot be rationalized in terms of simple point charges. ...
bYTEBoss introduction
... Conserved properties associated with C and P • C and P are still good symmetries in any reaction not involving the weak interaction – Can associate a conserved value with them (Noether Theorem) ...
... Conserved properties associated with C and P • C and P are still good symmetries in any reaction not involving the weak interaction – Can associate a conserved value with them (Noether Theorem) ...
Silicon-based Quantum Computation
... Scheme II: Shallow Donor Qubits II Overview The biggest problem with the original Kane computer proposal is the oscillatory behavior of the exchange interaction. In order to solve this problem, another approach was proposed [7], utilizing the same basic framework as the original Kane computer. Donor ...
... Scheme II: Shallow Donor Qubits II Overview The biggest problem with the original Kane computer proposal is the oscillatory behavior of the exchange interaction. In order to solve this problem, another approach was proposed [7], utilizing the same basic framework as the original Kane computer. Donor ...
Document
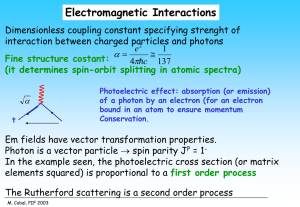
... very well confirmed by experiments partons are quarks Comparing proton and neutron structure functions and those from n scattering, e2a can be evaluated: it appears to be consistent with square charges of quarks. M. Cobal, PIF 2003 ...
... very well confirmed by experiments partons are quarks Comparing proton and neutron structure functions and those from n scattering, e2a can be evaluated: it appears to be consistent with square charges of quarks. M. Cobal, PIF 2003 ...
Quantum Theory of Angular Momentum and Atomic Structure
... If you let a quantum particle live on a unit sphere, “rotational energy” (L2 ) states are given by `(` + 1)~2 (` is a non-negative integer) and put the particle in an ` state, you can specify but one component of angular momentum precisely...the other two cannot be specif ied; also, the component ca ...
... If you let a quantum particle live on a unit sphere, “rotational energy” (L2 ) states are given by `(` + 1)~2 (` is a non-negative integer) and put the particle in an ` state, you can specify but one component of angular momentum precisely...the other two cannot be specif ied; also, the component ca ...