2. 2d Particle accelerators_tcm4-665527
... smallest known particles – the fundamental building blocks of all things. It will revolutionise our understanding, from the minuscule world deep within atoms to the vastness of the universe. Two beams of subatomic particles called ‘hadrons’ – either protons or lead ions – will travel in opposite dir ...
... smallest known particles – the fundamental building blocks of all things. It will revolutionise our understanding, from the minuscule world deep within atoms to the vastness of the universe. Two beams of subatomic particles called ‘hadrons’ – either protons or lead ions – will travel in opposite dir ...
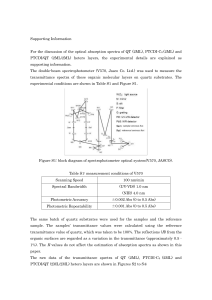
Partial widths of the Z
... We can identify (tag) jets originating from b quarks by looking for the electrons and muons coming from b decay. Naively expect 1/8th of decays to each type of lepton. Reality is close ; BR(be) = 10.9 % , BR(bm) = 10.9 % . But there are other sources of leptons in jets, such as K+ m+n, p0 ge+e- ...
... We can identify (tag) jets originating from b quarks by looking for the electrons and muons coming from b decay. Naively expect 1/8th of decays to each type of lepton. Reality is close ; BR(be) = 10.9 % , BR(bm) = 10.9 % . But there are other sources of leptons in jets, such as K+ m+n, p0 ge+e- ...
Lectures 6-7 - U of L Class Index
... where Δx is the uncertainty about position, Δp is the uncertainty about momentum (i.e. difference between maximum and minimum possible momentum values), and h is Planck’s constant. Scientists often use ħ to stand for h/2, so this formula can also be written as: ...
... where Δx is the uncertainty about position, Δp is the uncertainty about momentum (i.e. difference between maximum and minimum possible momentum values), and h is Planck’s constant. Scientists often use ħ to stand for h/2, so this formula can also be written as: ...
Computational Quantum Chemistry of Chemical Kinetic Modeling
... The aim of quantum chemistry is to provide a qualitative and quantitative description of molecular structure and the chemical properties of molecules. The principal theories considered in quantum chemistry are valence bond theory and molecular orbital theory. Valence bond theory has been proven to b ...
... The aim of quantum chemistry is to provide a qualitative and quantitative description of molecular structure and the chemical properties of molecules. The principal theories considered in quantum chemistry are valence bond theory and molecular orbital theory. Valence bond theory has been proven to b ...
SOLID-STATE PHYSICS II 2007 O. Entin-Wohlman
... momentum L = 3. This means that the states with J = 2, 3, and 4 are all possible. This gives for the case of n = 2 electrons 5 + 7 + 9 = 21 options. (Note that in this case, (2L + 1)(2S + 1) = 21.) However, Hund’s third rule tells us that the lowest energy is obtained for J = |L − S| = 2, and theref ...
... momentum L = 3. This means that the states with J = 2, 3, and 4 are all possible. This gives for the case of n = 2 electrons 5 + 7 + 9 = 21 options. (Note that in this case, (2L + 1)(2S + 1) = 21.) However, Hund’s third rule tells us that the lowest energy is obtained for J = |L − S| = 2, and theref ...
2000
... therefore the velocity of the impurity atoms was varied by the angle between the two laser beams. Collisions between the impurity atoms and the condensate were observed as a redistribution of momentum when the velocity distribution was analyzed with a ballistic expansion technique. The collisional c ...
... therefore the velocity of the impurity atoms was varied by the angle between the two laser beams. Collisions between the impurity atoms and the condensate were observed as a redistribution of momentum when the velocity distribution was analyzed with a ballistic expansion technique. The collisional c ...
Chen_APS 2006
... • A feasibility study has shown that it is practical to measure the radial electric field in the Helically Symmetric eXperiment, HSX, using ion beams. • Two options have been explored, a standard Heavy Ion Beam Probe, HIBP, and a system that measures the deflection of an ion beam due to the plasma e ...
... • A feasibility study has shown that it is practical to measure the radial electric field in the Helically Symmetric eXperiment, HSX, using ion beams. • Two options have been explored, a standard Heavy Ion Beam Probe, HIBP, and a system that measures the deflection of an ion beam due to the plasma e ...
PHY492: Nuclear & Particle Physics Lecture 18 Quark-onium QCD basics
... • Should be nine possible color-anticolor combinations ...
... • Should be nine possible color-anticolor combinations ...
F. J. García de Abajo, Phys. Rev. B 60, 6086
... variety of systems are investigated. A full curved-wave 共multipole兲 treatment of the electromagnetic field is used, in combination with improved iterative multiple-scattering techniques. The electron wave functions employed in the electron-diffraction analogy are replaced here by scalar functions th ...
... variety of systems are investigated. A full curved-wave 共multipole兲 treatment of the electromagnetic field is used, in combination with improved iterative multiple-scattering techniques. The electron wave functions employed in the electron-diffraction analogy are replaced here by scalar functions th ...
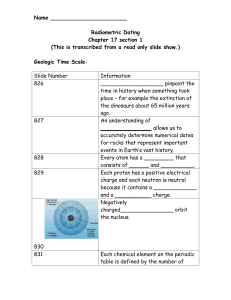
Geologic Time
... time in history when something took place – for example the extinction of the dinosaurs about 65 million years ago. An understanding of _______________ allows us to accurately determine numerical dates for rocks that represent important events in Earth’s vast history. Every atom has a _________ that ...
... time in history when something took place – for example the extinction of the dinosaurs about 65 million years ago. An understanding of _______________ allows us to accurately determine numerical dates for rocks that represent important events in Earth’s vast history. Every atom has a _________ that ...
Lecture note on Solid State Physics de Haas
... and solid lines correspond to the Landau levels of the electron and hole, respectively. The curve of EF vs B exhibits kinks at the fields where the Landau levels cross the Fermi energy. BCn±: the Landau level of the electron b- and c pockets with the quantum number n and the spin up (+) (down (-))-s ...
... and solid lines correspond to the Landau levels of the electron and hole, respectively. The curve of EF vs B exhibits kinks at the fields where the Landau levels cross the Fermi energy. BCn±: the Landau level of the electron b- and c pockets with the quantum number n and the spin up (+) (down (-))-s ...
The Nobel Prize in Physics 1901-2000
... in atoms and it was in this context that it could be put to its first crucial test. As early as 1896 Pieter Zeeman, who was looking for possible effects of electric and magnetic fields on light, made an important discovery namely, that spectral lines from sodium in a flame were split up into several ...
... in atoms and it was in this context that it could be put to its first crucial test. As early as 1896 Pieter Zeeman, who was looking for possible effects of electric and magnetic fields on light, made an important discovery namely, that spectral lines from sodium in a flame were split up into several ...
SSP Chapter 23
... Classically, because we know that the average energy of each electron is 3/ 2 kB T , the average will be zero, and because the kinetic energy is always positive, this implies that each electron will have exactly zero energy. Qua.ntum mechanically, the story is quite different. To begin with, E = 0 i ...
... Classically, because we know that the average energy of each electron is 3/ 2 kB T , the average will be zero, and because the kinetic energy is always positive, this implies that each electron will have exactly zero energy. Qua.ntum mechanically, the story is quite different. To begin with, E = 0 i ...
Emission Line Spectra and the Rydberg Constant
... number on the left refers to the principal quantum number, n, while the letter on the right refers to the angular momentum quantum number, l. This quantum number for any electron can range from 0 to n-1. For example, the 3s state on the para-Helium diagram refers to an electron with n = 3 and l = 0 ...
... number on the left refers to the principal quantum number, n, while the letter on the right refers to the angular momentum quantum number, l. This quantum number for any electron can range from 0 to n-1. For example, the 3s state on the para-Helium diagram refers to an electron with n = 3 and l = 0 ...
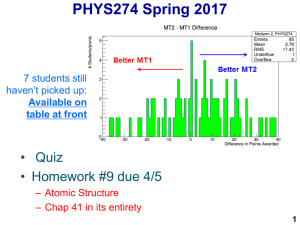
PHYSICS 113: Contemporary Physics – Midterm Exam Solution Key
... all of the complications that might arise for pushing for that long (the plane wouldn’t be long enough, air resistance would be insanely important, and so on). What is the momentum after 109 seconds? Sol. This is the exact same question as part b. We simply have: pf = F ∆t = 4N × 109 s = 4 × 109 J ( ...
... all of the complications that might arise for pushing for that long (the plane wouldn’t be long enough, air resistance would be insanely important, and so on). What is the momentum after 109 seconds? Sol. This is the exact same question as part b. We simply have: pf = F ∆t = 4N × 109 s = 4 × 109 J ( ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.