7.4 The Quantum-Mechanical Model of the Atom
... atom are called atomic orbitals • Born’s interpretation of the wavefunction – the probability to find the electron at a certain point (x, y, z) in space is proportional to the square of the wave function, Ψ2, in this point • Electron density diagrams – three-dimensional plots of the probability to f ...
... atom are called atomic orbitals • Born’s interpretation of the wavefunction – the probability to find the electron at a certain point (x, y, z) in space is proportional to the square of the wave function, Ψ2, in this point • Electron density diagrams – three-dimensional plots of the probability to f ...
1AMQ, Part II Quantum Mechanics
... acts to restrict the allowed energies (and hence other quantities eg. momentum, spin..) This force is specified by the potential, V(x,t). Example: Electrons in a 1-D Box Suppose an e- (in 1-d) is placed in a `box’ with impenetrable walls at x=0 and x=L. The electron can move freely (in x direction) ...
... acts to restrict the allowed energies (and hence other quantities eg. momentum, spin..) This force is specified by the potential, V(x,t). Example: Electrons in a 1-D Box Suppose an e- (in 1-d) is placed in a `box’ with impenetrable walls at x=0 and x=L. The electron can move freely (in x direction) ...
Radiation
... Force falls off with the square of distance k q1 q2 Twice as far: one quarter the force F = -----------r2 Three times as far: one ninth the force ...
... Force falls off with the square of distance k q1 q2 Twice as far: one quarter the force F = -----------r2 Three times as far: one ninth the force ...
Nobel Prize in Physics 1945 "for the discovery of the Exclusion
... - exclusion principle: No two electrons in an atom can exist in the same quantum state. Each electron must have a different set of quantum numbers n, l, ml, ms. - fundamental principle governing the electronic configuration in atoms discovered by Wolfgang Pauli in 1925 - principle found through the ...
... - exclusion principle: No two electrons in an atom can exist in the same quantum state. Each electron must have a different set of quantum numbers n, l, ml, ms. - fundamental principle governing the electronic configuration in atoms discovered by Wolfgang Pauli in 1925 - principle found through the ...
energy - Edublogs
... • Since the electrons’ energy are unique for each element, each element produces a unique spectra of colors when supplied energy. • We may see with our eyes only many overlaping colors of light. • To see all the distinct colors in the atom’s spectra requires a “diffraction grating”. ...
... • Since the electrons’ energy are unique for each element, each element produces a unique spectra of colors when supplied energy. • We may see with our eyes only many overlaping colors of light. • To see all the distinct colors in the atom’s spectra requires a “diffraction grating”. ...
January 2005
... forces. For N 1, what is the mean end-to-end length of the chain? What (Gaussian) probability distribution P (rN ) of the position rN of the free end of the polymer does this imply? ...
... forces. For N 1, what is the mean end-to-end length of the chain? What (Gaussian) probability distribution P (rN ) of the position rN of the free end of the polymer does this imply? ...
From the Big Bang to String Theory
... You can’t blame them. They knew they saw something. Saying “Arr! Check out yon sea-serpent!” sounds a lot better than “I don’t know what happens in a Black Hole.” Knowing which questions you can or cannot answer is just as important as the answers themselves. Right now, what we know about General Re ...
... You can’t blame them. They knew they saw something. Saying “Arr! Check out yon sea-serpent!” sounds a lot better than “I don’t know what happens in a Black Hole.” Knowing which questions you can or cannot answer is just as important as the answers themselves. Right now, what we know about General Re ...
Spin Current without Magnetic Material
... when you are finished with them. They are infinitely fudgable, and they have been used to fudge a broad array of new theories and maths since 1960. If all these theories and maths fell, dozens of living physicists would have to return their prizes to Stockholm. So we should expect the transparent mi ...
... when you are finished with them. They are infinitely fudgable, and they have been used to fudge a broad array of new theories and maths since 1960. If all these theories and maths fell, dozens of living physicists would have to return their prizes to Stockholm. So we should expect the transparent mi ...
Section 3: Modern Atomic Theory Atoms Section 3
... are called valence electrons, which determine the chemical properties of an atom. The diagram below shows how many electrons can be found in each of the first four energy levels of an atom. ...
... are called valence electrons, which determine the chemical properties of an atom. The diagram below shows how many electrons can be found in each of the first four energy levels of an atom. ...
Materiality: Is It Real?
... Mass is what comprises matter. It is the flip side of the energy manifestation of field. Its popularity as the basis of our material reality is the result of man’s practical nature. Physics is concerned with physical phenomena for which it is necessary to have practical explanations. Every physical ...
... Mass is what comprises matter. It is the flip side of the energy manifestation of field. Its popularity as the basis of our material reality is the result of man’s practical nature. Physics is concerned with physical phenomena for which it is necessary to have practical explanations. Every physical ...
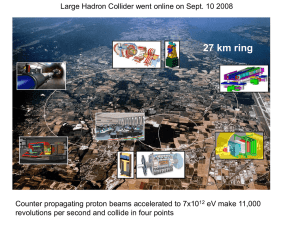
e - National Centre for Physics
... and a positively charged proton attract each other with a force which is proportional to their electric charges and inversely proportional to square of distance between them. But according to the concept of electromagnetic field introduced by Faraday and Maxwell ; presence of a charged particle prod ...
... and a positively charged proton attract each other with a force which is proportional to their electric charges and inversely proportional to square of distance between them. But according to the concept of electromagnetic field introduced by Faraday and Maxwell ; presence of a charged particle prod ...
Electron Configuration Notes File
... Steps to Writing Electron Configuration 1. Determine the # of electrons 2. Use the redesigned PT to get the configuration 3. Superscripts will equal the electrons ...
... Steps to Writing Electron Configuration 1. Determine the # of electrons 2. Use the redesigned PT to get the configuration 3. Superscripts will equal the electrons ...
1 Elementary Particle Mass-Radius Relationships S. Reucroft* and
... There are only two fundamental fields. These are gravity and electromagnetism and for both we use the classical 1/r2 relationships of Newton and Coulomb. In the case of gravity, we assume that the mass of a particle is its relativistic mass ...
... There are only two fundamental fields. These are gravity and electromagnetism and for both we use the classical 1/r2 relationships of Newton and Coulomb. In the case of gravity, we assume that the mass of a particle is its relativistic mass ...
Unit 5 Notes - Har
... Pauli exclusion principle – no two electrons in the same atom can have the same set of four quantum numbers (remember they must have different spins, but everything else may be the same) Example: ...
... Pauli exclusion principle – no two electrons in the same atom can have the same set of four quantum numbers (remember they must have different spins, but everything else may be the same) Example: ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.