Slide 1
... can plot a definite path for it which is called an orbit. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact inhabit regions of space known as orbitals. Orbits and orbitals sound similar, but t ...
... can plot a definite path for it which is called an orbit. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact inhabit regions of space known as orbitals. Orbits and orbitals sound similar, but t ...
10. Molecules and Solids
... molecules—the positive and negative charges both behave like point sources and so their fields cancel out perfectly! So how do molecules form? ...
... molecules—the positive and negative charges both behave like point sources and so their fields cancel out perfectly! So how do molecules form? ...
Work and Energy - FSU
... Motion With Constant Force: The work W done by a constant Force F~ whose point of application moves through a distance 4~x is defined to be W = F cos(θ) 4x where θ is the angle between the vector F~ and the vector 4~x, see figure 6-1 of Tipler-Mosca. If 4~x is along the x-axis, i.e. 4~x = 4x î = 4x ...
... Motion With Constant Force: The work W done by a constant Force F~ whose point of application moves through a distance 4~x is defined to be W = F cos(θ) 4x where θ is the angle between the vector F~ and the vector 4~x, see figure 6-1 of Tipler-Mosca. If 4~x is along the x-axis, i.e. 4~x = 4x î = 4x ...
The photoelectric effect - University of Toronto Physics
... - photoelectrons are emitted only if light frequency exceeds a threshold frequency fo. The value of fo depends on the type of cathode metal. - if the potential difference ΔV is made negative (anode negative with respect to the cathode), current I decreases until it becomes zero (at ΔV= -Vstop). Vsto ...
... - photoelectrons are emitted only if light frequency exceeds a threshold frequency fo. The value of fo depends on the type of cathode metal. - if the potential difference ΔV is made negative (anode negative with respect to the cathode), current I decreases until it becomes zero (at ΔV= -Vstop). Vsto ...
Conservation Laws
... of the end points F, FO. (Since these integrals are evaluated at a given time, they say nothing about what happens in the case of the actual displacement of a particle over the path, unless ...
... of the end points F, FO. (Since these integrals are evaluated at a given time, they say nothing about what happens in the case of the actual displacement of a particle over the path, unless ...
CHM 101 - Academic Computer Center
... Oxygen can form either covalent or ionic bonds. Explain the nature of each bond, the conditions under which each forms and the type of substances in each bond. ...
... Oxygen can form either covalent or ionic bonds. Explain the nature of each bond, the conditions under which each forms and the type of substances in each bond. ...
Measurement of the half-life of
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
History of Atomic Structure
... • Atoms of an element have the same size, mass, and chemical properties. • Atoms cannot be created, divided into smaller particles, or destroyed. • Atoms of different elements combine in whole number ratios to form compounds (H2O, CO2, MgO, etc.). • In a chemical reaction, atoms are separated, combi ...
... • Atoms of an element have the same size, mass, and chemical properties. • Atoms cannot be created, divided into smaller particles, or destroyed. • Atoms of different elements combine in whole number ratios to form compounds (H2O, CO2, MgO, etc.). • In a chemical reaction, atoms are separated, combi ...
Computational Spectroscopy
... Strategy is to model electron correlation via general functionals of the electron density. A functional is a function whose definition is also a function, that is, a function of a function. Hohenberg-Kohn theorem (Phys. Rev. 136, B864 (1964)) says that the ground state energy is equal to a functiona ...
... Strategy is to model electron correlation via general functionals of the electron density. A functional is a function whose definition is also a function, that is, a function of a function. Hohenberg-Kohn theorem (Phys. Rev. 136, B864 (1964)) says that the ground state energy is equal to a functiona ...
Slide 1
... III. The Bohr model for ___________________: 1. The 1 e- in H____________________ or __________ ____________the nucleus. It __________ in a ___________. 2. The e- can only be found at ______________ (certain specially allowed) distances, which are unfortunately still called __________________ . E ...
... III. The Bohr model for ___________________: 1. The 1 e- in H____________________ or __________ ____________the nucleus. It __________ in a ___________. 2. The e- can only be found at ______________ (certain specially allowed) distances, which are unfortunately still called __________________ . E ...
The History of the Atom
... are emitted by a radioactive source. They are large, heavy and positively charged. Beta Particles: high-energy, high-speed electrons or positrons emitted by a radioactive source. They are much smaller and lighter than Alpha Particles and are negatively charged. Gamma Radiation: electro magnetic radi ...
... are emitted by a radioactive source. They are large, heavy and positively charged. Beta Particles: high-energy, high-speed electrons or positrons emitted by a radioactive source. They are much smaller and lighter than Alpha Particles and are negatively charged. Gamma Radiation: electro magnetic radi ...
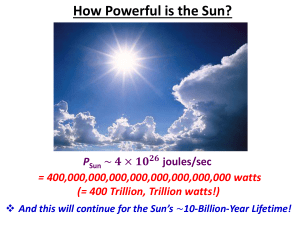
How Powerful is the Sun?
... And the tremendous penetrating power of Neutrinos helps us Study the Universe, today! ...
... And the tremendous penetrating power of Neutrinos helps us Study the Universe, today! ...
Cosmic Rays - High Energy Physics at Wayne State
... • We would expect that even if the muons are traveling at close to the speed of light, the average distance they would travel before decaying is 5 km. • i.e. they would not make it to sea level ...
... • We would expect that even if the muons are traveling at close to the speed of light, the average distance they would travel before decaying is 5 km. • i.e. they would not make it to sea level ...
File
... • Democritus was the first person to propose the existence of atoms. • According to Democritus, atoms are solid, homogenous, and indivisible. • Aristotle did not believe in the existence of atoms. • John Danton’s atomic theory is based on numerous scientific experiments. - Smallest particle of an el ...
... • Democritus was the first person to propose the existence of atoms. • According to Democritus, atoms are solid, homogenous, and indivisible. • Aristotle did not believe in the existence of atoms. • John Danton’s atomic theory is based on numerous scientific experiments. - Smallest particle of an el ...
What do the numbers 238, 235 written against the name of the
... interaction of neutrons in the incoming cosmic rays in the upper atmosphere with atmospheric nitrogen which yields carbon-14 and a proton. These constantly replenished carbon-14 atoms are soon oxidised by the atmospheric oxygen to become incorporated into the molecules of atmospheric carbon dioxide. ...
... interaction of neutrons in the incoming cosmic rays in the upper atmosphere with atmospheric nitrogen which yields carbon-14 and a proton. These constantly replenished carbon-14 atoms are soon oxidised by the atmospheric oxygen to become incorporated into the molecules of atmospheric carbon dioxide. ...
24. The Helium Atom
... two electrons behaves more or less independently, feeling the same force from the nucleus and having the same energy levels and definite-energy wavefunctions. I say “more or less,” because electrons are identical fermions, so even if they don’t exert any forces on each other, their combined wavefunc ...
... two electrons behaves more or less independently, feeling the same force from the nucleus and having the same energy levels and definite-energy wavefunctions. I say “more or less,” because electrons are identical fermions, so even if they don’t exert any forces on each other, their combined wavefunc ...
Scientific Notation - Warren County Public Schools
... Label each picture as precise, accurate, both, or neither. ...
... Label each picture as precise, accurate, both, or neither. ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.