Figure 7.18 The 3d orbitals
... - Instrumental techniques used to obtain information about atomic or molecular energy levels - Emission: electrons in an atom are excited to a higher energy state and then emit photons as they return to lower energy states - Absorption: electrons in an atom absorb photons of certain wavelengths and ...
... - Instrumental techniques used to obtain information about atomic or molecular energy levels - Emission: electrons in an atom are excited to a higher energy state and then emit photons as they return to lower energy states - Absorption: electrons in an atom absorb photons of certain wavelengths and ...
pptx
... GW-BSE: what is it for? DFT is a ground-state theory for electrons But many processes involve exciting electrons: • Transport of electrons, electron energy levels • Excited electrons ...
... GW-BSE: what is it for? DFT is a ground-state theory for electrons But many processes involve exciting electrons: • Transport of electrons, electron energy levels • Excited electrons ...
11.1 Nuclear Reactions
... neutrons are more likely to undergo β decay to convert a proton to a neutron. • Unstable isotopes having more neutrons than protons are more likely to undergo either positron emission or electron capture to convert a neutron to a proton. ...
... neutrons are more likely to undergo β decay to convert a proton to a neutron. • Unstable isotopes having more neutrons than protons are more likely to undergo either positron emission or electron capture to convert a neutron to a proton. ...
Chemistry Chapter 3
... • Atomic number called the Z number • Atomic mass is the average of the atomic masses of the naturally occurring isotopes of the element – Isotopes are atoms from same element having different numbers of neutrons ...
... • Atomic number called the Z number • Atomic mass is the average of the atomic masses of the naturally occurring isotopes of the element – Isotopes are atoms from same element having different numbers of neutrons ...
Chapter 8: Periodic Properties of the Elements
... Coulombs Law: F = kQ1Q2/d2 Since Energy is force times distance, E = kQ1Q2/d Coulombs Law describes attractions and repulsions between charged particles. Attraction is stronger as atomic sizes decrease and charge differences increase. Effective Nuclear Charge: Negatively charged electrons are attrac ...
... Coulombs Law: F = kQ1Q2/d2 Since Energy is force times distance, E = kQ1Q2/d Coulombs Law describes attractions and repulsions between charged particles. Attraction is stronger as atomic sizes decrease and charge differences increase. Effective Nuclear Charge: Negatively charged electrons are attrac ...
Advanced Characterization methods lectures
... e CN A where me is the electron mass, c is the speed of light, 0 is the free space permittivity, L is the path length and e is the electron charge. Finally, one can show that the relation to the dipole moment defined earlier is 8 2 me ...
... e CN A where me is the electron mass, c is the speed of light, 0 is the free space permittivity, L is the path length and e is the electron charge. Finally, one can show that the relation to the dipole moment defined earlier is 8 2 me ...
Firefly-On-Demand
... produced in association with lightning, b) the optical signature of the lightning flash, and c) the radio waves radiated by the lightning. TGFs are of inherent interest because they result from the most powerful natural particle acceleration process on Earth, in which thermal electrons are energized ...
... produced in association with lightning, b) the optical signature of the lightning flash, and c) the radio waves radiated by the lightning. TGFs are of inherent interest because they result from the most powerful natural particle acceleration process on Earth, in which thermal electrons are energized ...
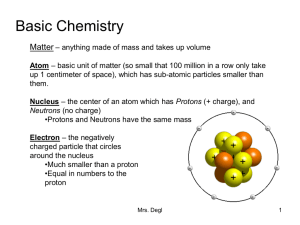
Basic Chemistry
... Basic Chemistry Matter – anything made of mass and takes up volume Atom – basic unit of matter (so small that 100 million in a row only take up 1 centimeter of space), which has sub-atomic particles smaller than them. Nucleus – the center of an atom which has Protons (+ charge), and Neutrons (no cha ...
... Basic Chemistry Matter – anything made of mass and takes up volume Atom – basic unit of matter (so small that 100 million in a row only take up 1 centimeter of space), which has sub-atomic particles smaller than them. Nucleus – the center of an atom which has Protons (+ charge), and Neutrons (no cha ...
Energy Band Diagrams - West Virginia University
... • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they carry current • Th ...
... • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they carry current • Th ...
Document
... of this nucleus causes it to undergo violent oscillations The 236U* nucleus becomes highly elongated, and the force of repulsion between the protons tends to increase the distortion The nucleus splits into two fragments, emitting several neutrons in the process ...
... of this nucleus causes it to undergo violent oscillations The 236U* nucleus becomes highly elongated, and the force of repulsion between the protons tends to increase the distortion The nucleus splits into two fragments, emitting several neutrons in the process ...
do physics online from quanta to quarks the bohr model of the atom
... An atom emits or absorbs energy only when an electron moves from one stable state to another. In a transition from its initial state to its final state, a photon is either emitted or absorbed and the energy of the photon is equal to the difference in the energy of the two states (equation 2) ...
... An atom emits or absorbs energy only when an electron moves from one stable state to another. In a transition from its initial state to its final state, a photon is either emitted or absorbed and the energy of the photon is equal to the difference in the energy of the two states (equation 2) ...
Molecular Geometry Why?
... is based on the premise that electrons around a central atom repel each other. Electron domains are areas of high electron density such as bonds (single, double or triple) and lone-pairs of electrons. In simple terms VSEPR means that all electron bonding domains and electron nonbonding domains aroun ...
... is based on the premise that electrons around a central atom repel each other. Electron domains are areas of high electron density such as bonds (single, double or triple) and lone-pairs of electrons. In simple terms VSEPR means that all electron bonding domains and electron nonbonding domains aroun ...
Entanglement of Identical Particles
... In quantum entanglement, two particles are correlated in such a way that any action on one of them affects the other even when they are far apart. The traditional methods of measuring the degree of quantum entanglement were originally developed for nonidentical particles, such as between an electron ...
... In quantum entanglement, two particles are correlated in such a way that any action on one of them affects the other even when they are far apart. The traditional methods of measuring the degree of quantum entanglement were originally developed for nonidentical particles, such as between an electron ...
UNIT 7 ATOMIC AND NUCLEAR PHYSICS
... of ordinary matter that has the properties of a chemical element. The typical picture of the atom (right) showing electrons orbiting around the nucleus results from the analogy with the planets orbiting around the sun. However, this simple picture turns out to be in serious con ...
... of ordinary matter that has the properties of a chemical element. The typical picture of the atom (right) showing electrons orbiting around the nucleus results from the analogy with the planets orbiting around the sun. However, this simple picture turns out to be in serious con ...
Quantum Computing with Electrons Floating on Liquid Helium P. M. Platzman
... would solve problems that could never be solved by any classical digital computer, even ones that were hundreds of billions of times larger and faster than current ones (1, 2). An AQC is made up of N interacting quantum objects called quantum bits (qubits), which have at least two quantum states (3) ...
... would solve problems that could never be solved by any classical digital computer, even ones that were hundreds of billions of times larger and faster than current ones (1, 2). An AQC is made up of N interacting quantum objects called quantum bits (qubits), which have at least two quantum states (3) ...
RelativityWorkbook-Student
... In our apparatus we can control the momentum of the particles by adjusting a magnetic field. We will also measure the time the particles take to travel a known distance, which gives us the velocity. Using this we can find what velocities correspond to what momenta. This will allow us to compare the ...
... In our apparatus we can control the momentum of the particles by adjusting a magnetic field. We will also measure the time the particles take to travel a known distance, which gives us the velocity. Using this we can find what velocities correspond to what momenta. This will allow us to compare the ...
Multi-electron atoms
... What’s different for these cases? Potential energy (V) changes! (Now more protons AND other electrons) V (for q1) = kqnucleusq1/rn-1 + kq2q1/r2-1 + kq3q1/r3-1 + …. Need to account for all the interactions among the electrons Must solve for all electrons at once! (use matrices) Gets very difficult to ...
... What’s different for these cases? Potential energy (V) changes! (Now more protons AND other electrons) V (for q1) = kqnucleusq1/rn-1 + kq2q1/r2-1 + kq3q1/r3-1 + …. Need to account for all the interactions among the electrons Must solve for all electrons at once! (use matrices) Gets very difficult to ...
atu_p_galla - Arkansas Space Grant Consortium
... chambers. Experimental evidence consistently showed propagation speeds approaching the speed of light. The speeds were shown to be supersonic even in comparison with electron acoustic speeds and thus, were called shock waves. The phenomenon involving no mass motion was concluded to be from electron ...
... chambers. Experimental evidence consistently showed propagation speeds approaching the speed of light. The speeds were shown to be supersonic even in comparison with electron acoustic speeds and thus, were called shock waves. The phenomenon involving no mass motion was concluded to be from electron ...
Chapter7 - FSU Chemistry
... n is a positive whole number, quantum number h is a constant " is the frequency of light emitted ...
... n is a positive whole number, quantum number h is a constant " is the frequency of light emitted ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.