Atomic Theory Review - hrsbstaff.ednet.ns.ca
... Both Rutherford’s and Bohr’s models of the atom have a nucleus, which is an extremely small, dense region in the center of the atom, that contains most of the atom’s mass and all of its positive charge. Both models have negatively charged electrons orbiting the nucleus. The difference is that Bohr’s ...
... Both Rutherford’s and Bohr’s models of the atom have a nucleus, which is an extremely small, dense region in the center of the atom, that contains most of the atom’s mass and all of its positive charge. Both models have negatively charged electrons orbiting the nucleus. The difference is that Bohr’s ...
Atomic Theory Review
... The letters s, p, d, or f, are used to designate a particular __ within an energy level. ...
... The letters s, p, d, or f, are used to designate a particular __ within an energy level. ...
Case 2 - Nikhef
... Classically, light behaves light waves. However, if you shoot light, photon per photon, it “comes in lumps”, just like electrons. Quantum Mechanics: for photons it is the same story as for electrons. ...
... Classically, light behaves light waves. However, if you shoot light, photon per photon, it “comes in lumps”, just like electrons. Quantum Mechanics: for photons it is the same story as for electrons. ...
atomicspectra1-2
... • A particular level is denoted either by nlj or by nl 2LJ with L = l and J = j. • The multiplicity of the L term is equal to 2S + 1 = 2s + 1 = 2.: doublet : two levels, with J = L ± 1/2, respectively • The Coulomb interaction between the nucleus and the single electron is dominant, so that the larg ...
... • A particular level is denoted either by nlj or by nl 2LJ with L = l and J = j. • The multiplicity of the L term is equal to 2S + 1 = 2s + 1 = 2.: doublet : two levels, with J = L ± 1/2, respectively • The Coulomb interaction between the nucleus and the single electron is dominant, so that the larg ...
idenfication and extraction of nuclear energy
... The name material point taken from classical mechanics refers to a particle of substance, not to a particle of matter which is a more general notion. Matter can be substance (the gravitational mass in mechanics) and radiation (the photon’s mass in electrodynamics). 60. The physical quantities. The p ...
... The name material point taken from classical mechanics refers to a particle of substance, not to a particle of matter which is a more general notion. Matter can be substance (the gravitational mass in mechanics) and radiation (the photon’s mass in electrodynamics). 60. The physical quantities. The p ...
1. dia
... In the presence of an external magnetic field, these different states will have different energies due to having different orientations of the magnetic dipoles in the external field, so the atomic energy levels are split into a larger number of levels and the spectral lines are also split. The rate ...
... In the presence of an external magnetic field, these different states will have different energies due to having different orientations of the magnetic dipoles in the external field, so the atomic energy levels are split into a larger number of levels and the spectral lines are also split. The rate ...
Quantum Master Equation of a Particle in a Gas Environment.
... where no is the density of environmental particles, ,g" is their momentum distribution; do denotes the differential cross-section of their scattering on the Brownian particle, while k f i= = kf- ki is the difference between the final and initial momenta of the scattered particle. Further symbols are ...
... where no is the density of environmental particles, ,g" is their momentum distribution; do denotes the differential cross-section of their scattering on the Brownian particle, while k f i= = kf- ki is the difference between the final and initial momenta of the scattered particle. Further symbols are ...
Models of Light Student Worksheet
... glucose which moves to wherever glucose is being used. The tracer emits a positron (the antimatter equivalent of an electron) which annihilates when it meets an electron. The mass of the particles is turned into the energy of two photons. These move off in opposite directions to conserve momentum. T ...
... glucose which moves to wherever glucose is being used. The tracer emits a positron (the antimatter equivalent of an electron) which annihilates when it meets an electron. The mass of the particles is turned into the energy of two photons. These move off in opposite directions to conserve momentum. T ...
Topic 3 Periodicity notes SL - Chemical Minds
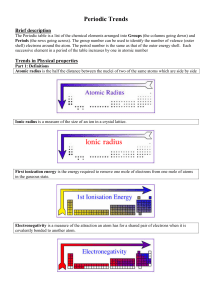
... Part 2: Physical properties down a group Going down a group, the atomic radius and ionic radius increase due to an increase in the number of electron shells surrounding the nucleus. The ionisation energy and electronegativity decrease because i) there is a decrease in the electrostatic attraction b ...
... Part 2: Physical properties down a group Going down a group, the atomic radius and ionic radius increase due to an increase in the number of electron shells surrounding the nucleus. The ionisation energy and electronegativity decrease because i) there is a decrease in the electrostatic attraction b ...
CHAP6
... between the particle-in-a-box system (infinite square well) and the Bohr’s hydrogen like atom? E.g. their energies level, their quantum number, their energy gap as a function of n, the sign of the energies, the potential etc. ...
... between the particle-in-a-box system (infinite square well) and the Bohr’s hydrogen like atom? E.g. their energies level, their quantum number, their energy gap as a function of n, the sign of the energies, the potential etc. ...
Lecture 5 Motion of a charged particle in a magnetic field
... Classically, in electric and magnetic field, particles experience a Lorentz force: F = q (E + v × B) q denotes charge (notation: q = −e for electron). Velocity-dependent force qv × B very different from that derived from scalar potential, and programme for transferring from classical to quantum mech ...
... Classically, in electric and magnetic field, particles experience a Lorentz force: F = q (E + v × B) q denotes charge (notation: q = −e for electron). Velocity-dependent force qv × B very different from that derived from scalar potential, and programme for transferring from classical to quantum mech ...
Name: Date ______ Introduction to Biochemistry Quiz
... 5. (2 points) ___________________ are formed when atoms share electrons, forming a very strong bond between those atoms. 6. (2 points) All ___________ is formed from basic building blocks called atoms. ...
... 5. (2 points) ___________________ are formed when atoms share electrons, forming a very strong bond between those atoms. 6. (2 points) All ___________ is formed from basic building blocks called atoms. ...
1 Non-exponential Auger decay A.M. Ishkhanyan and V.P. Krainov
... Because of quite depletion of the survival probability, the observation of nonexponential decay evolution at long timescales exceeding several lifetimes poses a major experimental challenge [5]. In addition, the interaction with the environment as well as several other possible factors, including th ...
... Because of quite depletion of the survival probability, the observation of nonexponential decay evolution at long timescales exceeding several lifetimes poses a major experimental challenge [5]. In addition, the interaction with the environment as well as several other possible factors, including th ...
Chapter 42
... Rutherford’s electrons are undergoing a centripetal acceleration. It should radiate electromagnetic waves of the same frequency. ...
... Rutherford’s electrons are undergoing a centripetal acceleration. It should radiate electromagnetic waves of the same frequency. ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.