
Lectuer 15
... - The z component of the angular momentum is determined completely by m through L z = m ħ. - The quantum number m is called the magnetic quantum number because the energy of a hydrogen atom in a magnetic field depends on m. - The (2 Ɩ + 1) – fold degeneracy in the absence of a magnetic field is spli ...
... - The z component of the angular momentum is determined completely by m through L z = m ħ. - The quantum number m is called the magnetic quantum number because the energy of a hydrogen atom in a magnetic field depends on m. - The (2 Ɩ + 1) – fold degeneracy in the absence of a magnetic field is spli ...
qftlect.dvi
... theory in dimensions d≥1. As we explained above, we have two main settings. 1. Minkowski space. Fields are functions on a spacetime VM , which is a real inner product space of signature (1, d —1). This is where physical processes actually "take place". The symmetry group of V, G = SO(1, d — 1), is c ...
... theory in dimensions d≥1. As we explained above, we have two main settings. 1. Minkowski space. Fields are functions on a spacetime VM , which is a real inner product space of signature (1, d —1). This is where physical processes actually "take place". The symmetry group of V, G = SO(1, d — 1), is c ...
2.4. Quantum Mechanical description of hydrogen atom
... Why is m called the magnetic quantum number? m determines the z component of the angular momentum. Since the electron is moving around the nuclei, and has a charge, it creates magnetic moment. There is a proportional relation between angular momentum and magnetic moment: ...
... Why is m called the magnetic quantum number? m determines the z component of the angular momentum. Since the electron is moving around the nuclei, and has a charge, it creates magnetic moment. There is a proportional relation between angular momentum and magnetic moment: ...
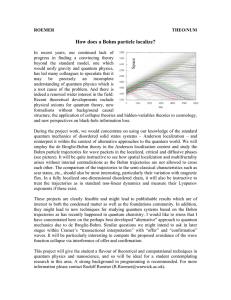
How does a Bohm particle localize?
... (see picture). It will be quite instructive to see how spatial localization and multifractality arises without internal contradictions as the Bohm trajectories are not allowed to cross each other. The comparison of the trajectories to the semi-classical characteristics such as scar states, etc., sho ...
... (see picture). It will be quite instructive to see how spatial localization and multifractality arises without internal contradictions as the Bohm trajectories are not allowed to cross each other. The comparison of the trajectories to the semi-classical characteristics such as scar states, etc., sho ...
Posttest for Uncertainty Principle Part 1
... (a) If you measure the square of the orbital angular momentum and obtained the value corresponding to quantum number 1 , what is the orbital angular momentum part of the state of the system after the measurement? Does the z-component of the orbital angular momentum have a definite value in this ...
... (a) If you measure the square of the orbital angular momentum and obtained the value corresponding to quantum number 1 , what is the orbital angular momentum part of the state of the system after the measurement? Does the z-component of the orbital angular momentum have a definite value in this ...
Lecture 26 Relevant sections in text: §3.6, 3.7 Two spin 1/2 systems
... We see that m = −1, 0, 1 with m = 0 being doubly degenerate (exercise). From our general results on angular momentum it is clear that the only possible values for the total spin quantum number are s = 0, 1. From this we can infer that the m = ±1 eigenvectors must be S2 eigenvectors with s = 1, but w ...
... We see that m = −1, 0, 1 with m = 0 being doubly degenerate (exercise). From our general results on angular momentum it is clear that the only possible values for the total spin quantum number are s = 0, 1. From this we can infer that the m = ±1 eigenvectors must be S2 eigenvectors with s = 1, but w ...
Lesson 1 - Tarleton State University
... Many lines were not seen. This indicated that there were selection rules that determined what lines were present. ...
... Many lines were not seen. This indicated that there were selection rules that determined what lines were present. ...













![1 Controlled Gates [6 points] 2 Finding a Function [14 Points]](http://s1.studyres.com/store/data/021376846_1-d8bb2ad3f9da11a441bbceaa64a9c875-300x300.png)








