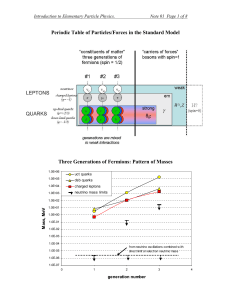
Periodic Table of Particles/Forces in the Standard Model
... quantum numbers like charge (electric, color, etc.), magnetic moment, etc. For photon , Z, and H, an anti-particle is the same as a particle. Same can be true for neutrinos, but we do not yet know this… In general, fermions—particles with half-integral spin: ½ , 3/2, …. Bosons—particles with integra ...
... quantum numbers like charge (electric, color, etc.), magnetic moment, etc. For photon , Z, and H, an anti-particle is the same as a particle. Same can be true for neutrinos, but we do not yet know this… In general, fermions—particles with half-integral spin: ½ , 3/2, …. Bosons—particles with integra ...
Slide 1
... 2) Y2 provides info about the electron’s location. 3) In the Quantum Mechanical Model, we speak of the probability (Y2) that the electron will be in a certain region of space at a given instant. 4) We call it probability density or electron density. ...
... 2) Y2 provides info about the electron’s location. 3) In the Quantum Mechanical Model, we speak of the probability (Y2) that the electron will be in a certain region of space at a given instant. 4) We call it probability density or electron density. ...
Chapter 1 Introduction
... force if µz > 0. Since the magnetic moment is proportional to the spin, in effect this device “measures” the spin of the particle. Now one could argue that the particle could take continuous values of the spin. So on a plate, suitable placed far away along the x axis, one should observe a big black ...
... force if µz > 0. Since the magnetic moment is proportional to the spin, in effect this device “measures” the spin of the particle. Now one could argue that the particle could take continuous values of the spin. So on a plate, suitable placed far away along the x axis, one should observe a big black ...
The Wave Nature of Matter - Waterford Public Schools
... • The square of a wave function (2) gives the probability of finding an electron in a particular infinitesimally small volume of space in an atom • Because we are treating electrons as waves (not particles), we cannot pinpoint the specific location of an electron! • Instead, mathematical solutions ...
... • The square of a wave function (2) gives the probability of finding an electron in a particular infinitesimally small volume of space in an atom • Because we are treating electrons as waves (not particles), we cannot pinpoint the specific location of an electron! • Instead, mathematical solutions ...
A quantum calculation of the higher order terms in the Bloch
... need to inverse equation (11). Taking Shirley’s notations ( b instead of &ol), we obtain : ...
... need to inverse equation (11). Taking Shirley’s notations ( b instead of &ol), we obtain : ...
Document
... vector or a unit vector if p’p=1. The m x 1 vectors p1, p2,…pn where n is less than or equal to m are said to be orthogonal if pi’pj=0 for all i not equal to j. If a group of n orthogonal vectors are also normalized, the vectors are said to be orthonormal. An m x m matrix consisting of orthonormal v ...
... vector or a unit vector if p’p=1. The m x 1 vectors p1, p2,…pn where n is less than or equal to m are said to be orthogonal if pi’pj=0 for all i not equal to j. If a group of n orthogonal vectors are also normalized, the vectors are said to be orthonormal. An m x m matrix consisting of orthonormal v ...