quantum mechanical methods for enzyme kinetics
... activation in two major ways. First, the electronic structure of the atoms that are involved in the bond-making and bond-breaking process should be treated quantum mechanically in calculating the PES that governs the motions that determine W(T, z). This is necessary because although traditional mole ...
... activation in two major ways. First, the electronic structure of the atoms that are involved in the bond-making and bond-breaking process should be treated quantum mechanically in calculating the PES that governs the motions that determine W(T, z). This is necessary because although traditional mole ...
Density functional theory and nuclear quantum effects
... This dissertation consists of two independent parts: density functional theory (Part I), and nuclear quantum effects (Part II). Kohn-Sham density functional theory (KSDFT) is by far the most widely used electronic structure theory in condensed matter systems. The computational time of KSDFT increase ...
... This dissertation consists of two independent parts: density functional theory (Part I), and nuclear quantum effects (Part II). Kohn-Sham density functional theory (KSDFT) is by far the most widely used electronic structure theory in condensed matter systems. The computational time of KSDFT increase ...
Imaging Electrons in Few-Electron Quantum Dots
... with the relevant energies. The tunnel barriers and the electrons in the leads are demonstrated by the dark and light orange areas respectively. Electrons in the leads have a Fermi distribution with a broadening around the Fermi energy EF given by kB T . The energy levels inside the quantum dot are ...
... with the relevant energies. The tunnel barriers and the electrons in the leads are demonstrated by the dark and light orange areas respectively. Electrons in the leads have a Fermi distribution with a broadening around the Fermi energy EF given by kB T . The energy levels inside the quantum dot are ...
Harvard University General Chemistry Practice Problems “The
... Gas is pumped out of the chamber until the total pressure is 0.100 torr. Calculate the new partial pressure of oxygen in the chamber. ...
... Gas is pumped out of the chamber until the total pressure is 0.100 torr. Calculate the new partial pressure of oxygen in the chamber. ...
Quantum information with Rydberg atoms
... 1 / R6 van der Waals forces at short range and 1 / R3 magnetic dipole-dipole forces beyond about 30 nm. At spacings greater than 1 m the interaction is weak, less than 1 Hz in frequency units, which implies that an array of neutral atom qubits can be structurally stable. On the other hand, excitati ...
... 1 / R6 van der Waals forces at short range and 1 / R3 magnetic dipole-dipole forces beyond about 30 nm. At spacings greater than 1 m the interaction is weak, less than 1 Hz in frequency units, which implies that an array of neutral atom qubits can be structurally stable. On the other hand, excitati ...
Transport study on two-dimensional electrons with controlled short-range alloy disorder
... (2DES). We then review the specific physics problems that we will investigate in later chapters of the thesis using these novel samples. In Chapter 2, we characterize all the 2DES samples, and look into the nature of the alloy disorder itself. We have measured the electron density, mobility and scat ...
... (2DES). We then review the specific physics problems that we will investigate in later chapters of the thesis using these novel samples. In Chapter 2, we characterize all the 2DES samples, and look into the nature of the alloy disorder itself. We have measured the electron density, mobility and scat ...
Interacting Rydberg atoms
... 1.1.2 Ramsey experiments . . . . . . . . . . . . . . . . . . . . 1.2 Rydberg atoms . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.2.1 Alkali Rydberg states . . . . . . . . . . . . . . . . . . . . 1.2.2 Calculation of wavefunctions and dipole matrix elements 1.2.3 Characteristics in electric ...
... 1.1.2 Ramsey experiments . . . . . . . . . . . . . . . . . . . . 1.2 Rydberg atoms . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.2.1 Alkali Rydberg states . . . . . . . . . . . . . . . . . . . . 1.2.2 Calculation of wavefunctions and dipole matrix elements 1.2.3 Characteristics in electric ...
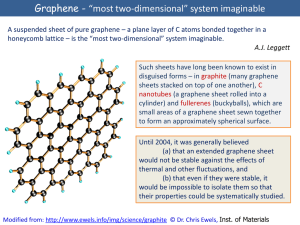
Graphene - âmost two-dimensionalâ system imaginable
... [1] K. S. Novoselov, A. K. Geim, S. V. Morosov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos, and A. A. Firsov, ...
... [1] K. S. Novoselov, A. K. Geim, S. V. Morosov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos, and A. A. Firsov, ...
Correlations and Counting Statistics of an Atom Laser
... innovative experiments by Hanbury Brown and Twiss, where they observed correlations of intensity fluctuations in two coherent beams of light [47, 48], the so-called bunching effect. Although initially measured with analog signals, in principle it represents the joint probability to detect two partic ...
... innovative experiments by Hanbury Brown and Twiss, where they observed correlations of intensity fluctuations in two coherent beams of light [47, 48], the so-called bunching effect. Although initially measured with analog signals, in principle it represents the joint probability to detect two partic ...
Quantum Physics of Atoms, Molecules, Solids, Nuclei, and Particles
... The basic purpose of this book is to present clear and valid treatments of the properties of almost all of the important quantum systems from the point of view of elementary quantum mechanics. Only as much quantum mechanics is developed as is required to accomplish the purpose. Thus we have chosen t ...
... The basic purpose of this book is to present clear and valid treatments of the properties of almost all of the important quantum systems from the point of view of elementary quantum mechanics. Only as much quantum mechanics is developed as is required to accomplish the purpose. Thus we have chosen t ...
Homework 5-7 answers
... C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 ...
... C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 ...
hydrogen storage
... this process, a gas molecule interacts with several atoms at the surface of a solid. The interaction is composed of two terms: an attractive term, which diminishes with the distance between the molecule and the surface to the power of -6, and a repulsive term, which diminishes with distance to the p ...
... this process, a gas molecule interacts with several atoms at the surface of a solid. The interaction is composed of two terms: an attractive term, which diminishes with the distance between the molecule and the surface to the power of -6, and a repulsive term, which diminishes with distance to the p ...
6 Chemical Bonding – Orbital Theory
... The orbitals containing a pair of electrons are not capable of combination. In fact, half-filled orbitals on one atom have a tendency to combine with half-filled orbitals on other atom, and the resulting orbital acquires a pair of electrons of opposite spins. (2) The atoms with valence or bonding or ...
... The orbitals containing a pair of electrons are not capable of combination. In fact, half-filled orbitals on one atom have a tendency to combine with half-filled orbitals on other atom, and the resulting orbital acquires a pair of electrons of opposite spins. (2) The atoms with valence or bonding or ...
numerical simulations of strongly correlated electron and spin systems
... First of all, I would like to thank my advisor Professor Christopher Henley for his excellent guidance and support during my Ph.D. His dedication and commitment to scientific research are exemplary and have influenced me greatly. His vast breadth of knowledge, tremendous physical insights and unique ...
... First of all, I would like to thank my advisor Professor Christopher Henley for his excellent guidance and support during my Ph.D. His dedication and commitment to scientific research are exemplary and have influenced me greatly. His vast breadth of knowledge, tremendous physical insights and unique ...
Electronic Structure of Clusters
... Most applications of ab initio quantum chemistry involve expanding the wavefunction using a basis set of atomic orbitals. As the many-body problem is not analytically tractable in either classical or quantum mechanics, we can never obtain the exact wavefunction. However, the variation principle12 te ...
... Most applications of ab initio quantum chemistry involve expanding the wavefunction using a basis set of atomic orbitals. As the many-body problem is not analytically tractable in either classical or quantum mechanics, we can never obtain the exact wavefunction. However, the variation principle12 te ...
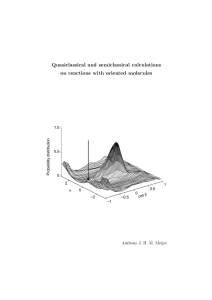
Quasiclassical and semiclassical calculations on reactions with oriented molecules cos β
... initio long range potential, which contains all ve asymptotically degenerate adiabatic potential energy surfaces. The construction of this potential is also reported in Chapter 3. In Chapter 4 the Ca + CH3 F reaction is reinvestigated using a semiclassical method, in which the electronic state of t ...
... initio long range potential, which contains all ve asymptotically degenerate adiabatic potential energy surfaces. The construction of this potential is also reported in Chapter 3. In Chapter 4 the Ca + CH3 F reaction is reinvestigated using a semiclassical method, in which the electronic state of t ...
Kondo physics in the single-electron transistor with ac driving Peter Nordlander
... by the time-averaged spectral density 具 dot( ⑀ ,t) 典 .13,14 For a given system, this average will depend on the driving amplitude V ac and frequency ⍀. In Fig. 2 we show the calculated 具 dot( ⑀ ,t) 典 as a function of energy ⑀ for a level with energy ⑀ dot(t)⫽ ⑀ dot⫹ ⑀ accos ⍀t at several differe ...
... by the time-averaged spectral density 具 dot( ⑀ ,t) 典 .13,14 For a given system, this average will depend on the driving amplitude V ac and frequency ⍀. In Fig. 2 we show the calculated 具 dot( ⑀ ,t) 典 as a function of energy ⑀ for a level with energy ⑀ dot(t)⫽ ⑀ dot⫹ ⑀ accos ⍀t at several differe ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.