topic 03 outline YT 2010 test
... B. The Bohr Atomic Model 1. Explaining the existence of line spectra The Bohr model was based on a simple postulate, Bohr applied to the hydrogen atom the concept that the electron can exist only in certain energy levels without an energy change but that, when the electron changes its state, it mu ...
... B. The Bohr Atomic Model 1. Explaining the existence of line spectra The Bohr model was based on a simple postulate, Bohr applied to the hydrogen atom the concept that the electron can exist only in certain energy levels without an energy change but that, when the electron changes its state, it mu ...
Slide 1
... waves can behave as particles and particles can behave as waves. This is called wave– particle duality. m = mass in kg p = momentum (mc) or (mv) h h For Light : = ...
... waves can behave as particles and particles can behave as waves. This is called wave– particle duality. m = mass in kg p = momentum (mc) or (mv) h h For Light : = ...
Year 11 Chemistry Balancing Equations
... Looking over your electron configurations, are there any elements above that have similar valence electron configurations to those of other elements? If so, list below the elements that are similar (in terms of valence electrons) and state the similarity for each of the groups. ...
... Looking over your electron configurations, are there any elements above that have similar valence electron configurations to those of other elements? If so, list below the elements that are similar (in terms of valence electrons) and state the similarity for each of the groups. ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... properties for a missing element of hypothetical element of the same family. Distinguish between atoms and ions (cations and anions and their formation) ...
... properties for a missing element of hypothetical element of the same family. Distinguish between atoms and ions (cations and anions and their formation) ...
Unit 16 Worksheet - Jensen Chemistry
... 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before it was ...
... 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before it was ...
1s 2s 2p - Solon City Schools
... embedded in a positively charged “pudding,” thus it was called the “plum pudding” model. ...
... embedded in a positively charged “pudding,” thus it was called the “plum pudding” model. ...
Quantum Mechanics: PHL555 Tutorial 2
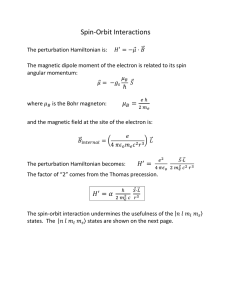
... fold degenerate first excited state split. 5. For a hydrogen atom placed in a weak uniform magnetic field along the , the Hamiltonian will be of the form ...
... fold degenerate first excited state split. 5. For a hydrogen atom placed in a weak uniform magnetic field along the , the Hamiltonian will be of the form ...
AP Exam Two Retake Qualifying Assignment
... What is the maximum number of d orbitals in a principal energy level? a. 1 c. 3 b. 2 d. 5 What is the maximum number of electrons in the fourth principal energy level? a. 2 c. 18 b. 8 d. 32 When an electron moves from a lower to a higher energy level, the electron ____. a. always doubles its energy ...
... What is the maximum number of d orbitals in a principal energy level? a. 1 c. 3 b. 2 d. 5 What is the maximum number of electrons in the fourth principal energy level? a. 2 c. 18 b. 8 d. 32 When an electron moves from a lower to a higher energy level, the electron ____. a. always doubles its energy ...
chapter02_part1_lecture - bloodhounds Incorporated
... • As radioactive isotopes decay, energy is released in the form of subatomic particles ...
... • As radioactive isotopes decay, energy is released in the form of subatomic particles ...
Electron Notes
... • One experiment involved the photoelectric effect, which refers to the emission of electrons from a metal when light shines on the metal. • This involved the frequency of the light. It was found that light was a form of energy that could knock an electron loose from a metal. ...
... • One experiment involved the photoelectric effect, which refers to the emission of electrons from a metal when light shines on the metal. • This involved the frequency of the light. It was found that light was a form of energy that could knock an electron loose from a metal. ...
Lecture (2) - MIT OpenCourseWare
... Since the ratio of Δx is large the ratio of masses will be large : m(-) ___________ m(+) This negative particle from the cathode ray tubes was named the electron (m = 9.11 x 10 ...
... Since the ratio of Δx is large the ratio of masses will be large : m(-) ___________ m(+) This negative particle from the cathode ray tubes was named the electron (m = 9.11 x 10 ...
pages 1-2 of the lecture notes
... Since the ratio of Δx is large the ratio of masses will be large : m(-) ___________ m(+) This negative particle from the cathode ray tubes was named the electron (m = 9.11 x 10 ...
... Since the ratio of Δx is large the ratio of masses will be large : m(-) ___________ m(+) This negative particle from the cathode ray tubes was named the electron (m = 9.11 x 10 ...
amu (atomic mass unit): a unit used to express very small masses
... sun. Bohr's first paper in this field dealt with the hydrogen atom, which he described as a single electron rotating in an orbit about a relatively heavy nucleus. He applied the concept of energy quanta, proposed in 1900 by the German physicist Max Planck (1858-1947) to the observed spectra of hydro ...
... sun. Bohr's first paper in this field dealt with the hydrogen atom, which he described as a single electron rotating in an orbit about a relatively heavy nucleus. He applied the concept of energy quanta, proposed in 1900 by the German physicist Max Planck (1858-1947) to the observed spectra of hydro ...
Where are the electrons
... Dual wave-particle nature of light • Einstein expanded on Planck’s theory and said that electromagnetic radiation has a dual wave-particle nature. • While light exhibits wave like properties, it can also be thought of as a stream of particles. • Each particle carries a quantum of energy – these par ...
... Dual wave-particle nature of light • Einstein expanded on Planck’s theory and said that electromagnetic radiation has a dual wave-particle nature. • While light exhibits wave like properties, it can also be thought of as a stream of particles. • Each particle carries a quantum of energy – these par ...
ch 11 - THE QUANTUM DEFECT - probs
... 11.2 A spherically symmetric singly charged positive ion (the "ionic core") is situated at the origin of coordinates. An electron is assumed to be at a fixed distance z along the z-axis. This electron induces in the ion a dipole moment, the magnitude of which is determined by the ion's polarizabilit ...
... 11.2 A spherically symmetric singly charged positive ion (the "ionic core") is situated at the origin of coordinates. An electron is assumed to be at a fixed distance z along the z-axis. This electron induces in the ion a dipole moment, the magnitude of which is determined by the ion's polarizabilit ...
Electrons and Atoms
... estimates the probability of finding an electron in a certain location • Energy is quantized - It comes in chunks. • A quantum is the amount of energy needed to move an electron from one energy level to another. • In 1926, Erwin Schrodinger derived an equation that described the energy and position ...
... estimates the probability of finding an electron in a certain location • Energy is quantized - It comes in chunks. • A quantum is the amount of energy needed to move an electron from one energy level to another. • In 1926, Erwin Schrodinger derived an equation that described the energy and position ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.