C:\SJWfiles\MyFirst Course\exam
... energy level to the perturbing potential. State the eigenvalues of the operator L z . B Z L , then derive Consider an atom with spin zero. If the perturbing potential is, e2m z e the energy shifts for states with orbital angular momentum, L 1. You should state the meaning for the various terms use ...
... energy level to the perturbing potential. State the eigenvalues of the operator L z . B Z L , then derive Consider an atom with spin zero. If the perturbing potential is, e2m z e the energy shifts for states with orbital angular momentum, L 1. You should state the meaning for the various terms use ...
Word - ASDL Community
... NMR SPECTROSCOPY – In-class Questions – Set 4– Nuclear Coupling 1. Consider the two hydrogen atoms HA and HB in the compound shown below. The X and Y groups are not hydrogen atoms and produce no magnetic fields. In particular, let’s focus on the effect that HB has on HA. We can think of HA “looking ...
... NMR SPECTROSCOPY – In-class Questions – Set 4– Nuclear Coupling 1. Consider the two hydrogen atoms HA and HB in the compound shown below. The X and Y groups are not hydrogen atoms and produce no magnetic fields. In particular, let’s focus on the effect that HB has on HA. We can think of HA “looking ...
Unit 6 Worksheet Package
... 2. Explain the properties of metals based on metallic bonding. 3. Write electron dot structures for representative atoms. 4. Draw electron dot structures for polyatomic ions. 5. Write electron dot structures for simple molecules. 6. Apply the octet rule to describe molecular structures. 7. List exce ...
... 2. Explain the properties of metals based on metallic bonding. 3. Write electron dot structures for representative atoms. 4. Draw electron dot structures for polyatomic ions. 5. Write electron dot structures for simple molecules. 6. Apply the octet rule to describe molecular structures. 7. List exce ...
Chemistry Final Exam Review 2006-2007
... 22. a. What are flame tests? b. What area of the electromagnetic radiation spectrum allows us to observe flame tests? c. Is energy released or absorbed when an electron falls from a higher energy level to a lower energy level? 23. What is the difference between a ground state and an excited state? 2 ...
... 22. a. What are flame tests? b. What area of the electromagnetic radiation spectrum allows us to observe flame tests? c. Is energy released or absorbed when an electron falls from a higher energy level to a lower energy level? 23. What is the difference between a ground state and an excited state? 2 ...
Chapter 2 - My Teacher Site
... When two atoms approach each other during a chemical reaction, only their electrons are involved directly ...
... When two atoms approach each other during a chemical reaction, only their electrons are involved directly ...
Physics 228, Lecture 12 Thursday, March 3, 2005 Uncertainty
... For light, which we treated classically as a wave, we knew what physical properties the wave represented — the electric and magnetic fields are functions of position and time, and these functions satisfy the wave equation. For matter, that is for things we treated classically as particles, we do not ...
... For light, which we treated classically as a wave, we knew what physical properties the wave represented — the electric and magnetic fields are functions of position and time, and these functions satisfy the wave equation. For matter, that is for things we treated classically as particles, we do not ...
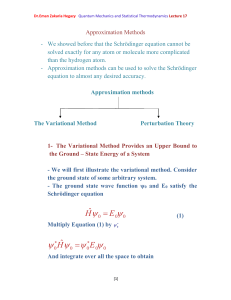
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... solved exactly for any atom or molecule more complicated than the hydrogen atom. - Approximation methods can be used to solve the Schrödinger equation to almost any desired accuracy. ...
... solved exactly for any atom or molecule more complicated than the hydrogen atom. - Approximation methods can be used to solve the Schrödinger equation to almost any desired accuracy. ...
Pdf
... provide a static negative charge cloud that is spatially distributed according to the extension of the orbitals comprised in (⫽0). The exchange operators reduce the magnitude of the corresponding repulsion somewhat, but only to a partial extent. As increases from 0 to 1 in H(), Eq. 共2.2兲, this ...
... provide a static negative charge cloud that is spatially distributed according to the extension of the orbitals comprised in (⫽0). The exchange operators reduce the magnitude of the corresponding repulsion somewhat, but only to a partial extent. As increases from 0 to 1 in H(), Eq. 共2.2兲, this ...
1. Review (MC problems, due Monday) 2. - mvhs
... (A) 4s and 6s electrons but different numbers of 4f electrons (B) 4s and 6s electrons but different number of 5s electrons (C) 4f and 6s electrons but different numbers of 5f electrons (D) 4f and 5s electrons but different numbers of 6s electrons (E) 5s electrons but different numbers of 6s and 4f e ...
... (A) 4s and 6s electrons but different numbers of 4f electrons (B) 4s and 6s electrons but different number of 5s electrons (C) 4f and 6s electrons but different numbers of 5f electrons (D) 4f and 5s electrons but different numbers of 6s electrons (E) 5s electrons but different numbers of 6s and 4f e ...
Quantum States of the- Trapped Electron for an Interstitial Ion*
... In the present calculation, the crystal is assumed to bc a dielectric medium and the net point charge for the interstitial ion is positive and unity. When the electron under discussion is moving in the region very close to the intcrstitial ion, its electric field is almost completely shielded by the ...
... In the present calculation, the crystal is assumed to bc a dielectric medium and the net point charge for the interstitial ion is positive and unity. When the electron under discussion is moving in the region very close to the intcrstitial ion, its electric field is almost completely shielded by the ...
The exotic world of quantum matter
... • Interacting quantum many-body systems (electrons, atoms, ..) condense into ordered states featuring spontaneous symmetry breaking and supporting a zoo of new “quasiparticles”. The search for new types of order in new (artificially synthesized) materials with novel properties not encountered in nat ...
... • Interacting quantum many-body systems (electrons, atoms, ..) condense into ordered states featuring spontaneous symmetry breaking and supporting a zoo of new “quasiparticles”. The search for new types of order in new (artificially synthesized) materials with novel properties not encountered in nat ...
5.1 Revising the Atomic Model
... 5.1 Revising the Atomic Model > Key Concepts Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations aroun ...
... 5.1 Revising the Atomic Model > Key Concepts Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations aroun ...
manual
... decreases as the scale of the box increases (Refer eq 4.37 at Oxtoby 7th ed). Since the decrease in kinetic energy is greater than the increase in potential energy, the effective potential energy decreases, and chemical bonding begins to occur. ...
... decreases as the scale of the box increases (Refer eq 4.37 at Oxtoby 7th ed). Since the decrease in kinetic energy is greater than the increase in potential energy, the effective potential energy decreases, and chemical bonding begins to occur. ...
Atomic Theory and Periodicity Questions
... The postulates of the Bohr model of the hydrogen atom can be stated as follows: (I) The electron can exist only in discrete states each with a definite energy. (II) The electron can exist only in certain circular orbits. (III) The angular momentum of the electron is nh/2 where n is any positive inte ...
... The postulates of the Bohr model of the hydrogen atom can be stated as follows: (I) The electron can exist only in discrete states each with a definite energy. (II) The electron can exist only in certain circular orbits. (III) The angular momentum of the electron is nh/2 where n is any positive inte ...
Introduction to Nanoscience
... A nanodevice that often appears in science fiction is a nanocamera. This is used to view the inside of the body or in other confined spaces where an ordinary camera would not fit. Unfortunately, it is not possible to make such a camera using conventional far field optics. Light sources and light det ...
... A nanodevice that often appears in science fiction is a nanocamera. This is used to view the inside of the body or in other confined spaces where an ordinary camera would not fit. Unfortunately, it is not possible to make such a camera using conventional far field optics. Light sources and light det ...
Department of Physics and Astronomy PhD Qualifying Examination
... If you have not previously passed any of the subject areas attempt any 2 of the 6 questions If you have previously passed one subject area attempt one of the 5 questions in the other areas. This is a closed book examination Start each question on a new sheet of paper – use only one side of each shee ...
... If you have not previously passed any of the subject areas attempt any 2 of the 6 questions If you have previously passed one subject area attempt one of the 5 questions in the other areas. This is a closed book examination Start each question on a new sheet of paper – use only one side of each shee ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.