E nergy spectra of quantum rings
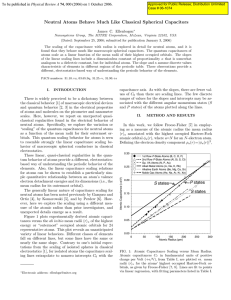
... arise by the pure presence of source and drain. In this case the probability densities start to oscillate around the ring’s circumference and a B-periodic Coulomb blockade amplitude can be understood this way. In reality the ring contains 2–3 radial modes and the ring potential will deviate from per ...
... arise by the pure presence of source and drain. In this case the probability densities start to oscillate around the ring’s circumference and a B-periodic Coulomb blockade amplitude can be understood this way. In reality the ring contains 2–3 radial modes and the ring potential will deviate from per ...
ppt
... growth direction and the QDs are distributed in the xy plane. PHYSICAL REVIEW B 77, 241304 ...
... growth direction and the QDs are distributed in the xy plane. PHYSICAL REVIEW B 77, 241304 ...
REsults
... We measured PL and PLE spectra of QWR with various gate voltages from 0 to 0.7V at 5K. Fig.2 (a) shows the normalized PL (dotted lines) and PLE (solid lines) spectra, where PL excitation energy is adjusted between 1.569 eV and 1.575eV. At low electron density (0V), the PLE spectrum is dominated by t ...
... We measured PL and PLE spectra of QWR with various gate voltages from 0 to 0.7V at 5K. Fig.2 (a) shows the normalized PL (dotted lines) and PLE (solid lines) spectra, where PL excitation energy is adjusted between 1.569 eV and 1.575eV. At low electron density (0V), the PLE spectrum is dominated by t ...
An introduction to the dynamical mean
... AIM and Kondo model were solved in 70s-80s by several techniques (renormalization group, Bethe anzatz, large N-expansion), other numerical and analytical methods are available now ...
... AIM and Kondo model were solved in 70s-80s by several techniques (renormalization group, Bethe anzatz, large N-expansion), other numerical and analytical methods are available now ...
Conservation of Energy in Classical Mechanics and Its Lack from the
... energy. A constant property of that parameter was at the basis of the whole mechanics of the examined system. As far as the motion of a celestial body—especially that present in the solar system—was examined, the theoretical framework based on the conservation property of energy seemed to be fully s ...
... energy. A constant property of that parameter was at the basis of the whole mechanics of the examined system. As far as the motion of a celestial body—especially that present in the solar system—was examined, the theoretical framework based on the conservation property of energy seemed to be fully s ...
File
... A. Electrons absorb energy as they move to an excited state. B. Electrons release energy as they move to an excited state. C. Electrons absorb energy as they return to the ground state. D. Electrons release energy as they return to the ground state. 6. Which statement regarding red and green visible ...
... A. Electrons absorb energy as they move to an excited state. B. Electrons release energy as they move to an excited state. C. Electrons absorb energy as they return to the ground state. D. Electrons release energy as they return to the ground state. 6. Which statement regarding red and green visible ...
Lecture 1 Review of hydrogen atom Heavy proton (put at the origin
... Let's go back to ground state of hydrogen: it has one proton with spin and one electron with spin (orbital angular momentum is zero). What is the total angular momentum of the hydrogen atom? ...
... Let's go back to ground state of hydrogen: it has one proton with spin and one electron with spin (orbital angular momentum is zero). What is the total angular momentum of the hydrogen atom? ...
Part IX
... The electrons in the valence bands are ~ in the bonds in r space. The valence electrons in the bonds have atomic-like character. (So, LCAO is a “natural” approximation for these). ...
... The electrons in the valence bands are ~ in the bonds in r space. The valence electrons in the bonds have atomic-like character. (So, LCAO is a “natural” approximation for these). ...
valence neutron
... • Over half the known nuclei have configurations (Z,N) even, J = 0+ • Recall that an empirical pairing term is included the semi-empirical mass formula to account for their unusual stability. N.B. The pairing term is not accounted for in the shell model, which ignores all interactions between parti ...
... • Over half the known nuclei have configurations (Z,N) even, J = 0+ • Recall that an empirical pairing term is included the semi-empirical mass formula to account for their unusual stability. N.B. The pairing term is not accounted for in the shell model, which ignores all interactions between parti ...
Ballistic Transport in a two-dimensional Electron System
... nearby the GaAs/AlGaAs interface. This is called a two-dimensional electron system (2DES). These electrons see only a small disturbing coulomb potential due to the ionized Si atoms far away from the conducting layer. Consequently they are able to travel typically some µm without being scattered. The ...
... nearby the GaAs/AlGaAs interface. This is called a two-dimensional electron system (2DES). These electrons see only a small disturbing coulomb potential due to the ionized Si atoms far away from the conducting layer. Consequently they are able to travel typically some µm without being scattered. The ...
50 Frequently Forgotten Facts
... a) Which statement best describes the reaction H + H H2 + energy: 1) A bond is being broken, which absorbs energy 2) A bond is being formed, which absorbs energy 3) A bond is being broken, which releases energy 4) A bond is being formed, which releases energy 37) Activation energy is the energy gi ...
... a) Which statement best describes the reaction H + H H2 + energy: 1) A bond is being broken, which absorbs energy 2) A bond is being formed, which absorbs energy 3) A bond is being broken, which releases energy 4) A bond is being formed, which releases energy 37) Activation energy is the energy gi ...
Dalton Model Reading
... Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). This marked the first truly scientific theory of the atom, since Dalton r ...
... Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). This marked the first truly scientific theory of the atom, since Dalton r ...
FREQUENTLY FORGOTTEN FACTS
... a) Which statement best describes the reaction H + H H2 + energy: 1) A bond is being broken, which absorbs energy 2) A bond is being formed, which absorbs energy 3) A bond is being broken, which releases energy 4) A bond is being formed, which releases energy 37) Activation energy is the energy gi ...
... a) Which statement best describes the reaction H + H H2 + energy: 1) A bond is being broken, which absorbs energy 2) A bond is being formed, which absorbs energy 3) A bond is being broken, which releases energy 4) A bond is being formed, which releases energy 37) Activation energy is the energy gi ...
Circularly Polarized Near-field Scanning Optical Microscope for
... of the apex of the probe tip. Furthermore, cares have been taken to avoid any stress or torsion by twisting or bending to minimize possible extrinsic birefringence in high magnetic field region using a dilution refrigerator specially designed for this purpose. This minimizes possible birefringence a ...
... of the apex of the probe tip. Furthermore, cares have been taken to avoid any stress or torsion by twisting or bending to minimize possible extrinsic birefringence in high magnetic field region using a dilution refrigerator specially designed for this purpose. This minimizes possible birefringence a ...
Visualizing the invisible nanoworld: ICT
... The corral image presents a more complex quantum landscape that includes a presentation of the wave-like nature of electrons. Again, it shows atoms as localized entities. They seem to stand out from an extended "sea" of wavelike corrugations which superpose to a circular pattern inside the ring of a ...
... The corral image presents a more complex quantum landscape that includes a presentation of the wave-like nature of electrons. Again, it shows atoms as localized entities. They seem to stand out from an extended "sea" of wavelike corrugations which superpose to a circular pattern inside the ring of a ...
Q 18.1–18.7 - DPG
... a high finesse optical resonator. While single-photon blockade has been demonstrated in such a system before [1], we here report on the first observation of two-photon blockade [2]. As a signature, we show a three-photon antibunching with simultaneous two-photon bunching observed in the light emitte ...
... a high finesse optical resonator. While single-photon blockade has been demonstrated in such a system before [1], we here report on the first observation of two-photon blockade [2]. As a signature, we show a three-photon antibunching with simultaneous two-photon bunching observed in the light emitte ...
5-1-light-quantized-energy
... of the element being examined and can be used to identify that element. O The fact the only certain colors appear in an element’s atomic emission spectrum means that only specific frequencies of light are emitted. O Those emitted frequencies are related to the energy formula Ephoton= hν, only photon ...
... of the element being examined and can be used to identify that element. O The fact the only certain colors appear in an element’s atomic emission spectrum means that only specific frequencies of light are emitted. O Those emitted frequencies are related to the energy formula Ephoton= hν, only photon ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.