chapter 5
... light is not enough to resolve (see) the detail structure of an atom as its size is only of the order of 100 nm. • 2) Most atoms are stable (i.e. atoms that are non radioactive) • 3) Atoms contain negatively charges, electrons, but are electrically neutral. An atom with Z electrons must also contain ...
... light is not enough to resolve (see) the detail structure of an atom as its size is only of the order of 100 nm. • 2) Most atoms are stable (i.e. atoms that are non radioactive) • 3) Atoms contain negatively charges, electrons, but are electrically neutral. An atom with Z electrons must also contain ...
Chapter 4 Spectroscopy
... 4.2 Atoms and Radiation Existence of spectral lines required new model of atom, so that only certain amounts of energy could be emitted or absorbed Bohr model had certain allowed orbits for electron ...
... 4.2 Atoms and Radiation Existence of spectral lines required new model of atom, so that only certain amounts of energy could be emitted or absorbed Bohr model had certain allowed orbits for electron ...
Light33i
... We have three equations and four unknowns. Need some other piece of information or some other relation. Bohr noted that Planck’s constant, h, had the units of angular momentum: L = mvr (kg*m2/sec = Joule*sec) so he tried this: L = nh ...
... We have three equations and four unknowns. Need some other piece of information or some other relation. Bohr noted that Planck’s constant, h, had the units of angular momentum: L = mvr (kg*m2/sec = Joule*sec) so he tried this: L = nh ...
Atomic mod
... an atom as its size is only of the order of 100 nm. • 2) Atoms are stable • 3) Atoms contain negatively charges, electrons, but are electrically neutral. An atom with Z electrons must also contain a net positive charge of +Ze. • 4) Atoms emit and absorb EM radiation (in other words, atoms interact w ...
... an atom as its size is only of the order of 100 nm. • 2) Atoms are stable • 3) Atoms contain negatively charges, electrons, but are electrically neutral. An atom with Z electrons must also contain a net positive charge of +Ze. • 4) Atoms emit and absorb EM radiation (in other words, atoms interact w ...
Test Review # 2 - Evan`s Chemistry Corner
... of atoms with more electrons. The wave mechanical model solved the problem. Thinking of the electron as a standing wave also helps to explain why the electron’s energy is quantized. The wave mechanical model describes the location of electrons a their most probable location rather than as orbits wit ...
... of atoms with more electrons. The wave mechanical model solved the problem. Thinking of the electron as a standing wave also helps to explain why the electron’s energy is quantized. The wave mechanical model describes the location of electrons a their most probable location rather than as orbits wit ...
QUANTUM NUMBERS
... The Bohr Atomic Theory BOHR MODEL (approx 1913) The Bohr model of the atom was based on the line spectra of the Hydrogen atom. The model also incorporated the concept developed by Einstein regarding the particle behaviour of light during emission or absorption (photon or quanta of energy). Postulate ...
... The Bohr Atomic Theory BOHR MODEL (approx 1913) The Bohr model of the atom was based on the line spectra of the Hydrogen atom. The model also incorporated the concept developed by Einstein regarding the particle behaviour of light during emission or absorption (photon or quanta of energy). Postulate ...
arty posters
... different places at the same time. But when you try to locate it with measuring instruments, the quantum object is suddenly reduced to one spot. This means that electrons, atoms, molecules, and even photons (light particles), are small particles and waves, both at the same time! ...
... different places at the same time. But when you try to locate it with measuring instruments, the quantum object is suddenly reduced to one spot. This means that electrons, atoms, molecules, and even photons (light particles), are small particles and waves, both at the same time! ...
Brief introduction to quantum mechanics
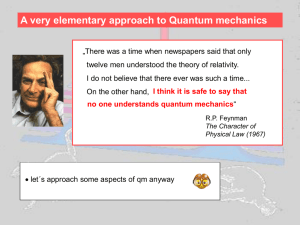
... A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood the theory of relativity. I do not believe that there ever was such a time... On the other hand, I think it is safe to say that no one understands quantum mechanics“ R.P. Feynman Th ...
... A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood the theory of relativity. I do not believe that there ever was such a time... On the other hand, I think it is safe to say that no one understands quantum mechanics“ R.P. Feynman Th ...
Frank-Hertz Experiment with Argon
... elegantly supported Niels Bohr's model of the atom, with electrons orbiting the nucleus with specific, discrete energies. Franck and Hertz were awarded the Nobel Prize in Physics in 1925 for this work. In the early 20th century, experiments by Ernest Rutherford established that atoms consisted of a ...
... elegantly supported Niels Bohr's model of the atom, with electrons orbiting the nucleus with specific, discrete energies. Franck and Hertz were awarded the Nobel Prize in Physics in 1925 for this work. In the early 20th century, experiments by Ernest Rutherford established that atoms consisted of a ...
RELATIVISTIC MOMENTUM AND ENERGY
... energy of an electron is 0.511 MeV, so there must be at least this much energy available in the decay. In a typical beta decay process, the kinetic energy of the electron is on the order of 1 MeV, so the electron must be treated relativistically. Another particle, called a neutrino, is also emitted ...
... energy of an electron is 0.511 MeV, so there must be at least this much energy available in the decay. In a typical beta decay process, the kinetic energy of the electron is on the order of 1 MeV, so the electron must be treated relativistically. Another particle, called a neutrino, is also emitted ...
Quantum Mechanics
... electrons can be represented by a single point charge at the mid-point between the nuclei. Calculate the magnitude this charge must have to ensure that the potential energy is negative. (b) A positive ion of kinetic energy 1 × 10−19 J collides with a stationary molecule of the same mass and forms a ...
... electrons can be represented by a single point charge at the mid-point between the nuclei. Calculate the magnitude this charge must have to ensure that the potential energy is negative. (b) A positive ion of kinetic energy 1 × 10−19 J collides with a stationary molecule of the same mass and forms a ...
Examination 3 Multiple Choice Questions
... Water has a composition of 11.2% Hydrogen and 88.8% Oxygen and a chemical formula of H2O. a) What mass of Oxygen is required to combine with 1.00g of Hydrogen? mass Water = 1.00 g / 0.112 = 8.93 g mass Oxygen = 8.93g - 1.00g = 7.93 g ...
... Water has a composition of 11.2% Hydrogen and 88.8% Oxygen and a chemical formula of H2O. a) What mass of Oxygen is required to combine with 1.00g of Hydrogen? mass Water = 1.00 g / 0.112 = 8.93 g mass Oxygen = 8.93g - 1.00g = 7.93 g ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).