PDF
... Definition 1.1. Let us recall that a quantum automaton is defined as a quantum algebraic topology object– the quantum triple QA = (G, H −
... Definition 1.1. Let us recall that a quantum automaton is defined as a quantum algebraic topology object– the quantum triple QA = (G, H −
Chemistry Final Exam Review 2006-2007
... 22. a. What are flame tests? b. What area of the electromagnetic radiation spectrum allows us to observe flame tests? c. Is energy released or absorbed when an electron falls from a higher energy level to a lower energy level? 23. What is the difference between a ground state and an excited state? 2 ...
... 22. a. What are flame tests? b. What area of the electromagnetic radiation spectrum allows us to observe flame tests? c. Is energy released or absorbed when an electron falls from a higher energy level to a lower energy level? 23. What is the difference between a ground state and an excited state? 2 ...
CHEM 101 Final (Term 151)
... 13. Perform the following mathematical operations and express the result in correct number of significant figures: ...
... 13. Perform the following mathematical operations and express the result in correct number of significant figures: ...
Evidencing `Tight Bound States` in the Hydrogen Atom
... particle-like spin, it is possible to justify Bohr’s physical assumptions and predict new properties of a real Dirac covariant polarized vacuum [23-26]. Bohr’s major contribution to modern physics was the model of photon emission-absorption in Hydrogen in terms of random energy jumps between stable ...
... particle-like spin, it is possible to justify Bohr’s physical assumptions and predict new properties of a real Dirac covariant polarized vacuum [23-26]. Bohr’s major contribution to modern physics was the model of photon emission-absorption in Hydrogen in terms of random energy jumps between stable ...
No Slide Title
... F central force in 3D movement of electron around nuclei movement of planets around sun For such systems L is a constant of motion, e.g. does not change with time since dL dt = 0 In quantum mechanics an operator O representing a constant of motion will commute with the Hamiltonian which means that w ...
... F central force in 3D movement of electron around nuclei movement of planets around sun For such systems L is a constant of motion, e.g. does not change with time since dL dt = 0 In quantum mechanics an operator O representing a constant of motion will commute with the Hamiltonian which means that w ...
Chapters 7, 8, 9 notes - SLCUSD Staff Directory
... There is a second way to describe electron configuration. It’s called _________________ notation, or circle notation. The advantage of this notation is that it can identify the number of _____________ electrons, which are important because they are involved in bonding, and determine the ____________ ...
... There is a second way to describe electron configuration. It’s called _________________ notation, or circle notation. The advantage of this notation is that it can identify the number of _____________ electrons, which are important because they are involved in bonding, and determine the ____________ ...
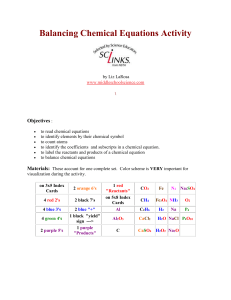
Balancing Chemical Equations Activity by Liz LaRosa www
... few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier ...
... few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier ...
Over 99% of the known mass of the universe is composed of two
... Over 99% of the known mass of the universe is composed of two particles: the proton and the neutron. Collectively known as the nucleon, they are the basic building blocks of all atomic nuclei. However, these ‘basic’ particles themselves possess a complex substructure. Our understanding of this struc ...
... Over 99% of the known mass of the universe is composed of two particles: the proton and the neutron. Collectively known as the nucleon, they are the basic building blocks of all atomic nuclei. However, these ‘basic’ particles themselves possess a complex substructure. Our understanding of this struc ...
Presentation #2
... function of time is called its trajectory. This trajectory is the full description of the motion of the particle Newton's Second Law enables us to calculate the trajectory of a particle in terms of the forces acting on it. Thus the entire history and the entire future of the body's motion, point by ...
... function of time is called its trajectory. This trajectory is the full description of the motion of the particle Newton's Second Law enables us to calculate the trajectory of a particle in terms of the forces acting on it. Thus the entire history and the entire future of the body's motion, point by ...
Unit 1: Building Blocks Homework
... Plants release ethene gas. A build up of ethene in florists shops will cause flowers to wither quickly. Florists can use a solid titanium dioxide catalyst to break down ethene gas to make flowers last longer. In the reaction, ethene reacts with the oxygen of the air to produce carbon ...
... Plants release ethene gas. A build up of ethene in florists shops will cause flowers to wither quickly. Florists can use a solid titanium dioxide catalyst to break down ethene gas to make flowers last longer. In the reaction, ethene reacts with the oxygen of the air to produce carbon ...
Coupling MOS Quantum Dot and Phosphorus Donor Qubit Systems
... Quantum computing has garnered significant attention due to the potential of significantly increasing computing efficiency. Si-MOS based quantum dot (QD) schemes are of particular interest due to their similarities to the mature technologies of the current semiconductor computing industry. Silicon a ...
... Quantum computing has garnered significant attention due to the potential of significantly increasing computing efficiency. Si-MOS based quantum dot (QD) schemes are of particular interest due to their similarities to the mature technologies of the current semiconductor computing industry. Silicon a ...
Problem Set - Appoquinimink High School
... b) What is the current measured in ammeter A? (2 points) c) How does potential difference across the 3 ohm resistor compare to the potential difference across the 4 ohm resistor? (1 point) 2) An electron is accelerated across a 1200 volt potential difference, as shown in the diagram. Upon reaching t ...
... b) What is the current measured in ammeter A? (2 points) c) How does potential difference across the 3 ohm resistor compare to the potential difference across the 4 ohm resistor? (1 point) 2) An electron is accelerated across a 1200 volt potential difference, as shown in the diagram. Upon reaching t ...
Introduction to PHY 855 “Introduction to field theory as it
... excitation of the electron field. /2/ Old way of thinking: A proton (or neutron) is a particle, or maybe a wave. New way of thinking: A proton (or neutron) is an excitation of the proton (or neutron) field. (Or, new new way of thinking: A nucleon is a bound state of quark and gluon ...
... excitation of the electron field. /2/ Old way of thinking: A proton (or neutron) is a particle, or maybe a wave. New way of thinking: A proton (or neutron) is an excitation of the proton (or neutron) field. (Or, new new way of thinking: A nucleon is a bound state of quark and gluon ...
PARTICLE IN AN INFINITE POTENTIAL WELL
... obtained from a qualitative analysis using the de Broglie principle. Observe that the lowest h2 state has an energy of 8mL 2 , which is different from zero. This is called the zero point energy. It implies that even when the system is in the ground state it is undergoing ceaseless motion. This zero ...
... obtained from a qualitative analysis using the de Broglie principle. Observe that the lowest h2 state has an energy of 8mL 2 , which is different from zero. This is called the zero point energy. It implies that even when the system is in the ground state it is undergoing ceaseless motion. This zero ...
Slide 1
... •The relationship between these levels of organization depends upon the energy state of the atom, which in turn depends upon a quantity called the principle quantum number. •Shell: Identified by a principal quantum number (1, 2, 3 . . . n) that specifies the energy level, or energy state, of the she ...
... •The relationship between these levels of organization depends upon the energy state of the atom, which in turn depends upon a quantity called the principle quantum number. •Shell: Identified by a principal quantum number (1, 2, 3 . . . n) that specifies the energy level, or energy state, of the she ...
Production of Phonon Schr ¨odinger Cat States in Benzoid Rings
... On the other hand, Schrödinger’s paper on the current situation of quantum mechanics, published in 1935[5], proposes a Gedankenexperiment to prepare superpositions of dead and alive cats, which highlights that the vast majority of states allowed by quantum mechanics are not observed in our macrosco ...
... On the other hand, Schrödinger’s paper on the current situation of quantum mechanics, published in 1935[5], proposes a Gedankenexperiment to prepare superpositions of dead and alive cats, which highlights that the vast majority of states allowed by quantum mechanics are not observed in our macrosco ...
Objectives Chapter 4
... • Quantum numbers specify the properties of atomic orbitals and the properties of electrons in orbitals. • The principal quantum number, symbolized by n, indicates the main energy level occupied by the electron. • The angular momentum quantum number, symbolized by l, indicates the shape of the orbit ...
... • Quantum numbers specify the properties of atomic orbitals and the properties of electrons in orbitals. • The principal quantum number, symbolized by n, indicates the main energy level occupied by the electron. • The angular momentum quantum number, symbolized by l, indicates the shape of the orbit ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).