ppt - vlsicad server (Prof. Markov`s group)
... Quantum Data • Classical bit – Two possible states: 0 or 1 – Measurement is straightforward ...
... Quantum Data • Classical bit – Two possible states: 0 or 1 – Measurement is straightforward ...
Atomic Structure and Periodicity – web
... HYDROGEN ATOMIC EMISSION SPECTRA • Niels Bohr connected spectra and the quantum ideas of ...
... HYDROGEN ATOMIC EMISSION SPECTRA • Niels Bohr connected spectra and the quantum ideas of ...
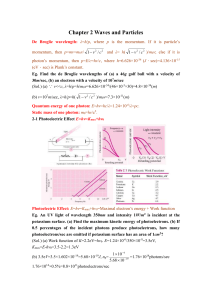
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
Tunneling spectroscopy of disordered two
... injection of an electron.10–12 This effect is well understood, and will not be modeled here. A quantitative estimate of the characteristic gap to the second Hubbard band VC00 can be obtained by assuming a specific form for the ground state in a specific valley. Assuming, for simplicity, radial symme ...
... injection of an electron.10–12 This effect is well understood, and will not be modeled here. A quantitative estimate of the characteristic gap to the second Hubbard band VC00 can be obtained by assuming a specific form for the ground state in a specific valley. Assuming, for simplicity, radial symme ...
A translation of" A New Solution to the Measurement Problem of
... The problems of definite outcomes and preferred basis are solved, but one question remains: while quantum mechanics derives superposition, why does the observer get single definite outcome? According to the free will theorem by Conway and Kochen and the discussion above, a reasonable answer will be: ...
... The problems of definite outcomes and preferred basis are solved, but one question remains: while quantum mechanics derives superposition, why does the observer get single definite outcome? According to the free will theorem by Conway and Kochen and the discussion above, a reasonable answer will be: ...
Reversible universal quantum computation within translation
... Introduce some noise, such that some of the states randomly turn into 1. Random state with probabilities ε for 1 and 1-ε for 0 . ...
... Introduce some noise, such that some of the states randomly turn into 1. Random state with probabilities ε for 1 and 1-ε for 0 . ...
Distributed measurement-based quantum computation
... computation has been known for quite some time through the teleportation protocol. Only much later it was realized that also in fault-tolerant constructions, measurements can be quite useful. Soon thereafter, with the advent of models such as the one-way quantum computer [RBB03] and the teleportatio ...
... computation has been known for quite some time through the teleportation protocol. Only much later it was realized that also in fault-tolerant constructions, measurements can be quite useful. Soon thereafter, with the advent of models such as the one-way quantum computer [RBB03] and the teleportatio ...
Equations - Pearson Schools and FE Colleges
... • In word equations, use only the chemical names of the substances • In chemical equations, correctly write numbers in formulae, e.g. H2O, not H2O or H2O • When balancing, do not change any chemical formula. Only put numbers in front of a formula. ...
... • In word equations, use only the chemical names of the substances • In chemical equations, correctly write numbers in formulae, e.g. H2O, not H2O or H2O • When balancing, do not change any chemical formula. Only put numbers in front of a formula. ...
apbio ch 2 study guide
... The periodic table of the elements shows the distribution of electrons in the first 18 elements from hydrogen to argon. o The elements are arranged in three rows or periods, corresponding to the number of electron shells in their atoms. o Elements in the same row have the same shells filled with ele ...
... The periodic table of the elements shows the distribution of electrons in the first 18 elements from hydrogen to argon. o The elements are arranged in three rows or periods, corresponding to the number of electron shells in their atoms. o Elements in the same row have the same shells filled with ele ...
Introduction - CNC Science
... The Molecular formula of a compound indicates the actual number of atoms of an each element present in the molecule of the compound ...
... The Molecular formula of a compound indicates the actual number of atoms of an each element present in the molecule of the compound ...
7. Radioactive decay
... At large distances, the lowest orders in this expansion are the only important ones. Thus, instead of considering the total radiation from a charge distribution, we can approximate it by considering the radiation arising from the first few multipoles: i.e. radiation from the electric dipole, the magn ...
... At large distances, the lowest orders in this expansion are the only important ones. Thus, instead of considering the total radiation from a charge distribution, we can approximate it by considering the radiation arising from the first few multipoles: i.e. radiation from the electric dipole, the magn ...
Determining g-factors in Rubidium-85 and Rubidium
... of the theoretical value of the ratio. This leads us to believe that a coil was connectedthat should not have been. If an unwanted coil was on this would mean that the cancellation of Earth’s magnetic field may not have been calibrated correctly leading to the incorrect slope values but a correct ra ...
... of the theoretical value of the ratio. This leads us to believe that a coil was connectedthat should not have been. If an unwanted coil was on this would mean that the cancellation of Earth’s magnetic field may not have been calibrated correctly leading to the incorrect slope values but a correct ra ...
Document
... never involved in the bond as they are too close to their own nucleus. 2 He atoms will never form a bond because Energy of He2 > 2 He. ...
... never involved in the bond as they are too close to their own nucleus. 2 He atoms will never form a bond because Energy of He2 > 2 He. ...
poster
... spread out over time. Therefore, the electron acts as a wave and will go through both slits and interfere with itself. This is why a distinct interference pattern will show up on the screen after shooting out electrons for a period of time.” Sample “Realist” Response “We just can't know EXACTLY wher ...
... spread out over time. Therefore, the electron acts as a wave and will go through both slits and interfere with itself. This is why a distinct interference pattern will show up on the screen after shooting out electrons for a period of time.” Sample “Realist” Response “We just can't know EXACTLY wher ...
Less than perfect wave functions in momentum-space
... New connections? (today’s talk) • Many of the most familiar 1D QM problems are based on potentials which are `less than perfect’ – Single δ(x), SW, quantum bouncer, etc. are singular – Finite wells are discontinuous V(X) – V(x) = F|x| has a discontinuous V’(x) ...
... New connections? (today’s talk) • Many of the most familiar 1D QM problems are based on potentials which are `less than perfect’ – Single δ(x), SW, quantum bouncer, etc. are singular – Finite wells are discontinuous V(X) – V(x) = F|x| has a discontinuous V’(x) ...
A First Look at Quantum Physics
... values, this means that if a given value of the angular momentum is allowed, its negative must also be allowed. (a) if L0 0 , then this criterion is satisfied, for L n (b) if L0 1 ...
... values, this means that if a given value of the angular momentum is allowed, its negative must also be allowed. (a) if L0 0 , then this criterion is satisfied, for L n (b) if L0 1 ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).