Document
... requires auxiliary conditions, related to a more detailed description of the system, for example locality of the transformations of the group in coordinate, momentum, or some other space, i.e., the requirement that the operators of the group have the form ...
... requires auxiliary conditions, related to a more detailed description of the system, for example locality of the transformations of the group in coordinate, momentum, or some other space, i.e., the requirement that the operators of the group have the form ...
Chapter 5 * Electronic Structure
... o s, p, d, and f sublevels o This version of the Periodic Table shows where the last electron will be located for each element. o Sodium’s last electron is in the s-sublevel o Helium’s last electron is in the s-sublevel. ...
... o s, p, d, and f sublevels o This version of the Periodic Table shows where the last electron will be located for each element. o Sodium’s last electron is in the s-sublevel o Helium’s last electron is in the s-sublevel. ...
balancing chemical equations worksheet
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
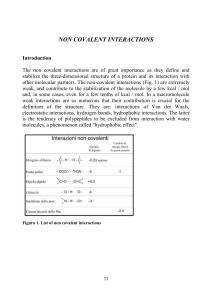
i principi di base - Structural Biology
... The hydrogen bond occurs between a donor and an acceptor of hydrogen atoms. When the interaction takes place between charged groups it is often referred to as salt bridge and it has properties typical either of an electrostatic interaction either of an hydrogen bond. The weak bonds between atoms wit ...
... The hydrogen bond occurs between a donor and an acceptor of hydrogen atoms. When the interaction takes place between charged groups it is often referred to as salt bridge and it has properties typical either of an electrostatic interaction either of an hydrogen bond. The weak bonds between atoms wit ...
Pearson Physics Level 30 Unit VIII Atomic Physics: Chapter 17
... 1. To be able to approach the nucleus, the particles must have tremendously high energy to overcome the electrostatic forces of repulsion within the nucleus. To probe the nucleus, the particles require even more energy to overcome the strong and weak nuclear forces. 2. Radioactive isotopes and cosmi ...
... 1. To be able to approach the nucleus, the particles must have tremendously high energy to overcome the electrostatic forces of repulsion within the nucleus. To probe the nucleus, the particles require even more energy to overcome the strong and weak nuclear forces. 2. Radioactive isotopes and cosmi ...
bilder/file/Quantum entanglement as a consequence
... theory which is both discrete and continuous at the same time but of course in different senses. This is essentially and indirectly echoing the same sentiment expressed by the present author long ago using the language of transfinite set theory [3-4]. Indeed in E-infinity theory we use Cantor sets w ...
... theory which is both discrete and continuous at the same time but of course in different senses. This is essentially and indirectly echoing the same sentiment expressed by the present author long ago using the language of transfinite set theory [3-4]. Indeed in E-infinity theory we use Cantor sets w ...
Comprehending Quantum Theory from Quantum Fields
... the basis of our daily physical reality are only secondary. They are excitations of their respective underlying quantum fields possessing propagating states of discrete energies, and it is these which constitute the primary reality. For example, an electron is the excitation of the abstract underlyi ...
... the basis of our daily physical reality are only secondary. They are excitations of their respective underlying quantum fields possessing propagating states of discrete energies, and it is these which constitute the primary reality. For example, an electron is the excitation of the abstract underlyi ...
CC_3_24.7.2013
... objects. However it became apparent in the 19th century, that classical physics fails to provide an accurate description of very small objects such as atoms and molecules. The effects of quantum mechanics are evident in many physical phenomena. In the late 1800’s and early 1900’s physicists identifi ...
... objects. However it became apparent in the 19th century, that classical physics fails to provide an accurate description of very small objects such as atoms and molecules. The effects of quantum mechanics are evident in many physical phenomena. In the late 1800’s and early 1900’s physicists identifi ...
Introduction, Introduction to lasers, Properties of light
... of mass would be moving) - this never happens in reality spontaneous emission has to be random - uniformly distributed over a 4p solid angle centered on the atom important consequences in laser cooling of atoms ...
... of mass would be moving) - this never happens in reality spontaneous emission has to be random - uniformly distributed over a 4p solid angle centered on the atom important consequences in laser cooling of atoms ...
3COM0074 Quantum Computing - Department of Computer Science
... will be tutorial sessions. These will give you week by week feed back on your progress with the mathematical strand of the course. The mathematical strand of this course will have an additional ½ hour set aside each week (at 9:00am prior to the Monday morning lecture ) to discuss problems that have ...
... will be tutorial sessions. These will give you week by week feed back on your progress with the mathematical strand of the course. The mathematical strand of this course will have an additional ½ hour set aside each week (at 9:00am prior to the Monday morning lecture ) to discuss problems that have ...
Magnetism - Bartol Research Institute
... • S is maximum • L is maximum consistent with S • J=|L-S| for less than half-filled; L+S for more than half filled. ...
... • S is maximum • L is maximum consistent with S • J=|L-S| for less than half-filled; L+S for more than half filled. ...
... to the orbit types of the group action, the manifold is stratified into different strata. Mechanics will be set up on each stratum and then reduced by symmetry. We apply this idea, taking M and G as the center-of-mass system for N bodies and the rotation group SO(3), respectively. The center-of-mass ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).