Topic 1 Review - Capital High School
... 34. The first ionization energies of the elements __________ as you go from left to right across a period of the periodic table, and __________ as you go from the bottom to the top of a group in the table. 35. In general, as you go across a period in the periodic table from left to right: (1) the at ...
... 34. The first ionization energies of the elements __________ as you go from left to right across a period of the periodic table, and __________ as you go from the bottom to the top of a group in the table. 35. In general, as you go across a period in the periodic table from left to right: (1) the at ...
Honors Chemistry / SAT II
... particles and electrons arranged in concentric shells around the nucleus.” This description most clearly fits the atomic theory proposed by (D) Thomson (A) Bohr (B) Rutherford (E) Avogadro (C) Dalton 2487. The maximum number of electrons possible in the second energy level of an atom is (D) 18 (A) 8 ...
... particles and electrons arranged in concentric shells around the nucleus.” This description most clearly fits the atomic theory proposed by (D) Thomson (A) Bohr (B) Rutherford (E) Avogadro (C) Dalton 2487. The maximum number of electrons possible in the second energy level of an atom is (D) 18 (A) 8 ...
Relation between the Gravitational and Magnetic Fields
... electron charge; the energy of this rotation or electromagnetic field energy gives rise to the mass. The charge is the time it takes in returning in the fourth dimension. Therefore, in the electron: • Rotation in the fourth dimension, which originates the electric charge and so, the electric field ...
... electron charge; the energy of this rotation or electromagnetic field energy gives rise to the mass. The charge is the time it takes in returning in the fourth dimension. Therefore, in the electron: • Rotation in the fourth dimension, which originates the electric charge and so, the electric field ...
Document
... technology even further by developing a smaller scale version that is capable of being put on an Earth-orbiting satellite for transmitting quantum keys distances of hundreds of miles between the satellite and a ground station. ...
... technology even further by developing a smaller scale version that is capable of being put on an Earth-orbiting satellite for transmitting quantum keys distances of hundreds of miles between the satellite and a ground station. ...
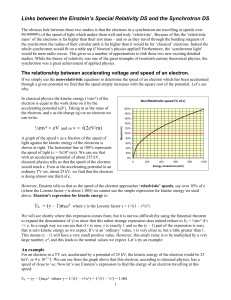
Links between the Einstein`s Special Relativity DS and
... magnets, wigglers and undulators. The bending magnets are uniform in field and produce a circular path to keep the electrons in the ring. The wigglers and undulators have alternating vertical fields which cause the electrons to oscillate in a horizontal plane. A obvious question arises: As the perio ...
... magnets, wigglers and undulators. The bending magnets are uniform in field and produce a circular path to keep the electrons in the ring. The wigglers and undulators have alternating vertical fields which cause the electrons to oscillate in a horizontal plane. A obvious question arises: As the perio ...
- Snistnote
... • Somerfield proposed the quantum free electron theory and he assumed that the valance electron are free in a metal piece and they obey quantum laws . • According to quantum theory the free electrons occupy different energy levels present in the metal. • According to this theory only Fermi level ele ...
... • Somerfield proposed the quantum free electron theory and he assumed that the valance electron are free in a metal piece and they obey quantum laws . • According to quantum theory the free electrons occupy different energy levels present in the metal. • According to this theory only Fermi level ele ...
berggren
... of nanotechnology as a whole. We are investigating fundamental challenges associated with continued scaling of electronic and nano-photonic device components. We are investigating the resolution limits of charged-particle lithography, including electron-beam and ion-beam lithography. The group also ...
... of nanotechnology as a whole. We are investigating fundamental challenges associated with continued scaling of electronic and nano-photonic device components. We are investigating the resolution limits of charged-particle lithography, including electron-beam and ion-beam lithography. The group also ...
Atomic Electron Configurations and Chapter 8 Chemical Periodicity
... positive charges. Plum-pudding model. ¾ 1911(Rutherford): Protons (positively charge) and neutrons (neutral) are located in the centre of the atom atom. Electrons are somewhere outside the nucleus. ¾ 1913 (Bohr): Electrons are moving in a circular orbit around the nucleus. Only certain orbits with f ...
... positive charges. Plum-pudding model. ¾ 1911(Rutherford): Protons (positively charge) and neutrons (neutral) are located in the centre of the atom atom. Electrons are somewhere outside the nucleus. ¾ 1913 (Bohr): Electrons are moving in a circular orbit around the nucleus. Only certain orbits with f ...
Course Syllabus Course Number and Name: CHM:160 Chemistry I
... 6. Assign quantum numbers to each electron in an atom and draw orbital diagrams utilizing Hund's Rule. 7. Derive orbital diagrams and quantum numbers for each electron in a monatomic ion. Unit Six At the end of this unit, the student will be able to: 1. Trace the development of the Periodic Table fr ...
... 6. Assign quantum numbers to each electron in an atom and draw orbital diagrams utilizing Hund's Rule. 7. Derive orbital diagrams and quantum numbers for each electron in a monatomic ion. Unit Six At the end of this unit, the student will be able to: 1. Trace the development of the Periodic Table fr ...
here - Foundations of Physics 2013
... In Newtonian QM the uncertainty present is self-locating uncertainty. One needs to determine which world they are in since many are consistent with any particular set of experiences. Since, in general, many worlds will be consistent with one’s experiences, each should be assigned equal credence ...
... In Newtonian QM the uncertainty present is self-locating uncertainty. One needs to determine which world they are in since many are consistent with any particular set of experiences. Since, in general, many worlds will be consistent with one’s experiences, each should be assigned equal credence ...
Conceptual Issues in Canonical Quantum Gravity and Cosmology
... This can be compared with the corresponding situation in electrodynamics: the Gauss constraint ∇E = 4πρ is preserved in time if and only if electric charge is conserved in time. The second theorem states: Einstein’s equations are the unique propagation law consistent with the constraints. Again, thi ...
... This can be compared with the corresponding situation in electrodynamics: the Gauss constraint ∇E = 4πρ is preserved in time if and only if electric charge is conserved in time. The second theorem states: Einstein’s equations are the unique propagation law consistent with the constraints. Again, thi ...
Quantization of Mechanical Motion
... Resonant tunneling is a complex phenomenon which compiles two quantum phenomena: quantum tunneling and quantum interference Propagation of electronic waves similar to that of ordinary waves experiences a set of multiple reflections moving back and forth between the barriers. The total amplitude to t ...
... Resonant tunneling is a complex phenomenon which compiles two quantum phenomena: quantum tunneling and quantum interference Propagation of electronic waves similar to that of ordinary waves experiences a set of multiple reflections moving back and forth between the barriers. The total amplitude to t ...
SPS 3
... The emitted photons are allowed to enter a Hanbury Brown-Twiss setup. The beamsplitter of the Hanbury Brown-Twiss setup directed about half of the incident photons to APD 1 and the other half to APD 2. These two APD's are used to compensate a dead time of each detector in measuring the time interval ...
... The emitted photons are allowed to enter a Hanbury Brown-Twiss setup. The beamsplitter of the Hanbury Brown-Twiss setup directed about half of the incident photons to APD 1 and the other half to APD 2. These two APD's are used to compensate a dead time of each detector in measuring the time interval ...
Physics League Across Nume ous Countries for Kick
... What does the latter situation mean, physically? Interestingly, LEEM does not only have a good lateral resolution. It is also an ideal probe to study the properties of layered materials in the vertical z-direction. The simplest example would be two layers of graphene (graphene is a hexagonal sheet o ...
... What does the latter situation mean, physically? Interestingly, LEEM does not only have a good lateral resolution. It is also an ideal probe to study the properties of layered materials in the vertical z-direction. The simplest example would be two layers of graphene (graphene is a hexagonal sheet o ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).