Chapter 12 - Midway ISD
... Takes shape of its container Properties can be explained by the kinetic molecular theory (KMT) just like gases were explained Fluid – has the ability to flow ...
... Takes shape of its container Properties can be explained by the kinetic molecular theory (KMT) just like gases were explained Fluid – has the ability to flow ...
PHASES OF MATTER -4 PHASE DIAGRAMS
... • Until point A the phase and temperature are both changes. Between B and C also the phase changes as the temperature increases. ...
... • Until point A the phase and temperature are both changes. Between B and C also the phase changes as the temperature increases. ...
Lecture 35 (Slides) November 7
... • 1. A total of 3.00 g of liquid water was placed in an evacuated (initially) 12.0L container maintained at a temperature of 80.0 0C. Will a liquid/gas equilibrium be established? What piece of data is required to solve this problem? If no liquid/gas equilibrium is established, determine how much ad ...
... • 1. A total of 3.00 g of liquid water was placed in an evacuated (initially) 12.0L container maintained at a temperature of 80.0 0C. Will a liquid/gas equilibrium be established? What piece of data is required to solve this problem? If no liquid/gas equilibrium is established, determine how much ad ...
Phy 211: General Physics I
... this is a consequence of Pascal’s Principle. At sea level the atmospheric pressure is ...
... this is a consequence of Pascal’s Principle. At sea level the atmospheric pressure is ...
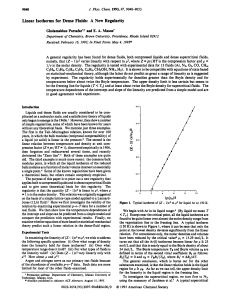
Linear Isotherms for Dense Fluids
... the thermal pressure. In order to model the experimental results, we should take m = 3 and n = 9 and have the last term of eq 5 be nearly constant in the density range of interest. This last expectation is somewhat surprising but is borne out for real fluids. For example, for Ar in the density range ...
... the thermal pressure. In order to model the experimental results, we should take m = 3 and n = 9 and have the last term of eq 5 be nearly constant in the density range of interest. This last expectation is somewhat surprising but is borne out for real fluids. For example, for Ar in the density range ...
Print Activity - Let`s Talk Science
... Is it a liquid or a solid? they do in a normal solid. This makes the goop feel and act as a solid. When the pressure is released the molecules form the random arrangement of a liquid and the goop suddenly flows. Why does it matter? All fluids have a measurable viscosity. Viscosity is the thickness ...
... Is it a liquid or a solid? they do in a normal solid. This makes the goop feel and act as a solid. When the pressure is released the molecules form the random arrangement of a liquid and the goop suddenly flows. Why does it matter? All fluids have a measurable viscosity. Viscosity is the thickness ...
FREEZING – is the change of a liquid to a solid. Freezing occurs
... Gas Laws (Charles’ Law) At constant pressure… The volume of gas varies directly with the temperature. Volume UP… Temperature UP Volume DOWN… Temperature DOWN ...
... Gas Laws (Charles’ Law) At constant pressure… The volume of gas varies directly with the temperature. Volume UP… Temperature UP Volume DOWN… Temperature DOWN ...
Carbon Dioxide Chemistry
... Carbon Dioxide Chemistry Most molecules can exist as gases, liquids and solids, depending on the temperature. For example if you heat ice, it becomes water, and if you heat water, it becomes a gas. Carbon dioxide is gaseous at room temperature. However, in much colder temperatures, it is a solid . ...
... Carbon Dioxide Chemistry Most molecules can exist as gases, liquids and solids, depending on the temperature. For example if you heat ice, it becomes water, and if you heat water, it becomes a gas. Carbon dioxide is gaseous at room temperature. However, in much colder temperatures, it is a solid . ...
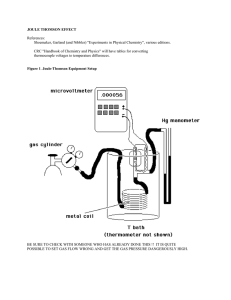
joule thomson effect
... Recommended gases are carbon dioxide and nitrogen. Hook up the carbon dioxide first (it shows the largest effect and is therefore easiest to work with at the star.) Make about 10 measurements between 50 and 200 kPa. For each pressure, set the pressure changes slowly, make sure that the magnetic stir ...
... Recommended gases are carbon dioxide and nitrogen. Hook up the carbon dioxide first (it shows the largest effect and is therefore easiest to work with at the star.) Make about 10 measurements between 50 and 200 kPa. For each pressure, set the pressure changes slowly, make sure that the magnetic stir ...
Acids and bases
... For example, the melting point of NaCl is 1073 K, but is lowered if CaCl2 is added as in the Downs process. In the solid state, HgCl2 forms a molecular lattice, and layer structures are adopted by HgBr2 (distorted CdI2 lattice) and HgI2. An important group of molten salts with more convenient operat ...
... For example, the melting point of NaCl is 1073 K, but is lowered if CaCl2 is added as in the Downs process. In the solid state, HgCl2 forms a molecular lattice, and layer structures are adopted by HgBr2 (distorted CdI2 lattice) and HgI2. An important group of molten salts with more convenient operat ...
States of Matter
... Variables are pressure (y) and temperature (x) Triple point: temperature and pressure where solid, liquid and gas can exist in equilibrium Critical temperature: temperature above which a substance cannot be liquified Critical pressure: pressure necessary to liquefy a substance at the critical temper ...
... Variables are pressure (y) and temperature (x) Triple point: temperature and pressure where solid, liquid and gas can exist in equilibrium Critical temperature: temperature above which a substance cannot be liquified Critical pressure: pressure necessary to liquefy a substance at the critical temper ...
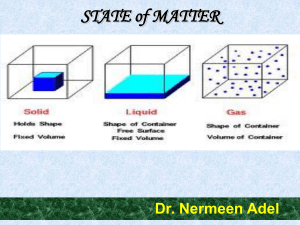
STATE of MATTER
... molecules) are PACKED CLOSELY together. The forces between them are STRONG enough so that they cannot move freely but can only vibrate. • A solid has a stable, definite shape, and a definite volume. • Solids can be changed into liquids by melting, and liquids can be transformed into solids by freezi ...
... molecules) are PACKED CLOSELY together. The forces between them are STRONG enough so that they cannot move freely but can only vibrate. • A solid has a stable, definite shape, and a definite volume. • Solids can be changed into liquids by melting, and liquids can be transformed into solids by freezi ...
Real Gases
... slow. In supercritical fluids, which have a lot of space between molecules, diffusion of solutes is much faster that in ordinary liquids and the viscosity is much lower than in ordinary liquids. Moreover, the properties of supercritical fluids in the region near the critical point vary very rapidly ...
... slow. In supercritical fluids, which have a lot of space between molecules, diffusion of solutes is much faster that in ordinary liquids and the viscosity is much lower than in ordinary liquids. Moreover, the properties of supercritical fluids in the region near the critical point vary very rapidly ...
Word
... b. Water boils above 100 0C at higher pressures c. Water boils below 100 0C at lower pressures C. Condensation 1. The conversion of a gas to a liquid by the removal of energy IV. Freezing and Melting A. Freezing Point 1. The temperature at which the solid and liquid are in equilibrium at 1 atm 2. Fo ...
... b. Water boils above 100 0C at higher pressures c. Water boils below 100 0C at lower pressures C. Condensation 1. The conversion of a gas to a liquid by the removal of energy IV. Freezing and Melting A. Freezing Point 1. The temperature at which the solid and liquid are in equilibrium at 1 atm 2. Fo ...
Pre-AP Chemistry Kinetic Theory and Heat Quiz
... coexist. The critical point is the point at which phases of matter __become ill defined__. The critical temperature is the temperature above which what cannot happen? a gas cannot be compressed into a liquid The critical pressure is the pressure it takes to _compress a gas into a liquid at the criti ...
... coexist. The critical point is the point at which phases of matter __become ill defined__. The critical temperature is the temperature above which what cannot happen? a gas cannot be compressed into a liquid The critical pressure is the pressure it takes to _compress a gas into a liquid at the criti ...
Lecture 1
... Zeroth law of thermodynamics - If systems A and B are in thermal equilibrium and systems B and C are also in thermal equilibrium, then A and C should be in thermal equilibrium when brought into thermal contact. ...
... Zeroth law of thermodynamics - If systems A and B are in thermal equilibrium and systems B and C are also in thermal equilibrium, then A and C should be in thermal equilibrium when brought into thermal contact. ...
ln2_storage_pre
... On the other hand, the gas cylinders are probably at room temperature. This is way above the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the criti ...
... On the other hand, the gas cylinders are probably at room temperature. This is way above the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the criti ...
Precipitation of fluticasone propionate microparticles using
... and expand or extract organic solvents and as result lower their solvation power, makes it possible the use of SCFs for the precipitation of solids from organic solutions. The process could be the injection of a solution of the substrate in an organic solvent into a vessel which is swept by a superc ...
... and expand or extract organic solvents and as result lower their solvation power, makes it possible the use of SCFs for the precipitation of solids from organic solutions. The process could be the injection of a solution of the substrate in an organic solvent into a vessel which is swept by a superc ...
CYL110
... Soluble components are extracted from a substrate by a high pressure gas, and the extracted components that have been dissolved in the gas are precipitated from the gas when the pressure is reduced. ...
... Soluble components are extracted from a substrate by a high pressure gas, and the extracted components that have been dissolved in the gas are precipitated from the gas when the pressure is reduced. ...
Understanding physical rock properties and their relation to fluid
... The electrical conductivity of rocks is, in addition to lithological factors (mineralogy, porosity) and physical parameters (temperature, pressure) sensitive to the nature of pore fluids (phase, salinity), and thus may be an indicative measure for fluid-rock interactions. Especially near the critica ...
... The electrical conductivity of rocks is, in addition to lithological factors (mineralogy, porosity) and physical parameters (temperature, pressure) sensitive to the nature of pore fluids (phase, salinity), and thus may be an indicative measure for fluid-rock interactions. Especially near the critica ...
Supercritical fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be ""fine-tuned"". Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes. Carbon dioxide and water are the most commonly used supercritical fluids, being used for decaffeination and power generation, respectively.