Waves & Oscillations Physics 42200 Spring 2013 Semester Matthew Jones
... “It's of no use whatsoever … this is just an experiment that proves Maestro Maxwell was right—we just have these mysterious electromagnetic waves that we cannot see with the naked eye. But they are there.” Asked about the ramifications of his discoveries, Hertz replied, "Nothing, I guess." ...
... “It's of no use whatsoever … this is just an experiment that proves Maestro Maxwell was right—we just have these mysterious electromagnetic waves that we cannot see with the naked eye. But they are there.” Asked about the ramifications of his discoveries, Hertz replied, "Nothing, I guess." ...
mindful universe - Thedivineconspiracy.org
... way in this struggle, just as they do. But it cannot help him without being in some way efficacious and influencing the course of his bodily history.” James went on to examine the circumstances under which consciousness appears, and ended up saying: “The conclusion that it is useful is, after all this, ...
... way in this struggle, just as they do. But it cannot help him without being in some way efficacious and influencing the course of his bodily history.” James went on to examine the circumstances under which consciousness appears, and ended up saying: “The conclusion that it is useful is, after all this, ...
Chapter 9 The Atom - Bakersfield College
... the wave function (y). The square of the wave function (y2) is called the probability density. For a given object, the greater the probability density at a certain time and place, the greater the likelihood of finding the object there at that time. The de Broglie waves of a moving object are in the ...
... the wave function (y). The square of the wave function (y2) is called the probability density. For a given object, the greater the probability density at a certain time and place, the greater the likelihood of finding the object there at that time. The de Broglie waves of a moving object are in the ...
Quantum Harmonic Oscillator
... times 1/2, 3/2, 5/2, and so forth. This is a feature of many quantum mechanical systems. In the following section on ladder operators, we will engage in a more detailed examination of this phenomenon. Secondly, the lowest achievable energy is not zero, but , which is called the "ground state energy" ...
... times 1/2, 3/2, 5/2, and so forth. This is a feature of many quantum mechanical systems. In the following section on ladder operators, we will engage in a more detailed examination of this phenomenon. Secondly, the lowest achievable energy is not zero, but , which is called the "ground state energy" ...
INCONSISTENT HISTORIES REVEALED BY QUANTUM
... – Hence, it could not interact with the other atom and should not be entangled with it. – But, by violating Bell’s inequality, its “having preserved its photon” is due to entanglement with the ...
... – Hence, it could not interact with the other atom and should not be entangled with it. – But, by violating Bell’s inequality, its “having preserved its photon” is due to entanglement with the ...
pdf
... the later stages of both courses. The two slides shown in Figure 2 are illustrative of how the differences between the two courses could be more subtle, yet still significant. Both slides summarize the results for the system referred to in PHYS3A as the Infinite Square Well, but which Instructor B c ...
... the later stages of both courses. The two slides shown in Figure 2 are illustrative of how the differences between the two courses could be more subtle, yet still significant. Both slides summarize the results for the system referred to in PHYS3A as the Infinite Square Well, but which Instructor B c ...
Quantum Number, n. - Lyndhurst Schools
... • Schrödinger’s equation requires 3 quantum numbers: 1. Principal Quantum Number, n. This is the same as Bohr’s n. As n becomes larger, the atom becomes larger and the electron is further from the nucleus. ( n = 1 , 2 , 3 , 4 , …. ) 2. Angular Momentum Quantum Number, . This quantum number depends ...
... • Schrödinger’s equation requires 3 quantum numbers: 1. Principal Quantum Number, n. This is the same as Bohr’s n. As n becomes larger, the atom becomes larger and the electron is further from the nucleus. ( n = 1 , 2 , 3 , 4 , …. ) 2. Angular Momentum Quantum Number, . This quantum number depends ...
Chapter 7, Quantum Nos.
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
Quantum mechanics is the theory that we use to describe the
... observable, of a system. This is unlike classical and relativistic theories, where everything exists with precise and definite values, and the time evolution of a system can theoretically be determined as far into the future as we want. This is not the case in quantum mechanics; we can only define p ...
... observable, of a system. This is unlike classical and relativistic theories, where everything exists with precise and definite values, and the time evolution of a system can theoretically be determined as far into the future as we want. This is not the case in quantum mechanics; we can only define p ...
Atomic spectra and the Bohr atom
... of same n by giving them different shapes; any integer value from 0 to n-1; orbitals of same n but different l are in different sub-shells: s p d f g ...
... of same n by giving them different shapes; any integer value from 0 to n-1; orbitals of same n but different l are in different sub-shells: s p d f g ...
Chp7,Quantum_Num
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
Slides1 - University of Guelph
... • To understand from basic principles how a quantum information protocol works in theory and in practice using optics • I chose quantum teleportation where we can understand the discrete (polarization) and continuous variable versions of this ...
... • To understand from basic principles how a quantum information protocol works in theory and in practice using optics • I chose quantum teleportation where we can understand the discrete (polarization) and continuous variable versions of this ...
Section 2 Notes
... In Conclusion: The first three quantum numbers: the principal quantum (n), the angular quantum number (l) and the magnetic quantum number (m) are integers. The principal quantum number (n) cannot be zero: n must be 1, 2, 3, etc. The angular quantum number (l) can be any integer between 0 and (n – 1) ...
... In Conclusion: The first three quantum numbers: the principal quantum (n), the angular quantum number (l) and the magnetic quantum number (m) are integers. The principal quantum number (n) cannot be zero: n must be 1, 2, 3, etc. The angular quantum number (l) can be any integer between 0 and (n – 1) ...
Key Challenges for Theoretical Computer Science
... Find limits of computationally sound interactive proofs, which prove a statement by performing a computation that would be infeasible if the statement were false. ...
... Find limits of computationally sound interactive proofs, which prove a statement by performing a computation that would be infeasible if the statement were false. ...
Precedence and freedom in quantum physics
... that the result of an individual measurement on elements of an entangled system could not be predicted by any knowledge of the past. An entangled state can be novel in that it can be formed from a composition of subsystems into a state never before occurring in the prior history of the universe. Th ...
... that the result of an individual measurement on elements of an entangled system could not be predicted by any knowledge of the past. An entangled state can be novel in that it can be formed from a composition of subsystems into a state never before occurring in the prior history of the universe. Th ...
Quantum Information Science and Technology
... don’t electrons spiral into the nucleus of an atom? -this quantum formalism predicts all sorts of weird and non-intuitive things… e.g., the EPR thought experiment -1990’s… when technology evolves to the Copyright 2001 S.D. Personick, All rights reserved point where experiments can be ...
... don’t electrons spiral into the nucleus of an atom? -this quantum formalism predicts all sorts of weird and non-intuitive things… e.g., the EPR thought experiment -1990’s… when technology evolves to the Copyright 2001 S.D. Personick, All rights reserved point where experiments can be ...
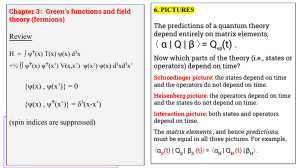
α | Q | β 〉= Q (t) . 〈 Review
... where H0 is solvable and H1 is a set of interactions, possibly having small effects. {Usually H0 is a single particle operator; and H1 is a two-particle operator describing the interactions between particles.} ...
... where H0 is solvable and H1 is a set of interactions, possibly having small effects. {Usually H0 is a single particle operator; and H1 is a two-particle operator describing the interactions between particles.} ...