* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Formatting Blackline Masters
Rutherford backscattering spectrometry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Crystallization wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical reaction wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Chemical equilibrium wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Process chemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Electrochemistry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Biochemistry wikipedia , lookup
Water splitting wikipedia , lookup
Institute of Chemistry Ceylon wikipedia , lookup
History of molecular theory wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Drug discovery wikipedia , lookup
Click chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Electrolysis of water wikipedia , lookup
History of chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Analytical chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Green chemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
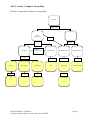
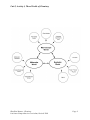
Unit 1, Activity 2, Specific Assessment Rubric Chemistry Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 1 Unit 1, Activity 2, Specific Assessment Rubric 3 Measurements are to the correct number of significant figures Units included Answers are within the range of acceptable error Measurements finished within the prescribed time limit Questions Answered 2 0 All measurements 2 or 3 measurements Less than 2 measurements All measurements 2 or 3 measurements Less than 2 measurements All measurements 2 or 3 measurements Less than 2 measurements All measurements All safety rules followed Answered correctly 2 or 3 measurements Less than 2 measurements Answered incorrectly but supported by evidence Answered incorrectly. No supporting evidence. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 1 Unit 1, Activity 3, Accuracy and Precision Worksheet Figure 1 Figure 2 Figure 3 1. Determine the accuracy and precision represented by each group of darts in the figures above. Explain your choices using complete sentences. Figure 1 Figure 2 Figure 3 Precision? Accuracy? 2. A basketball player throws 100 free-throws; 95 of these balls go through the goal; 5 miss the goal entirely. Describe the precision and accuracy of the free-throws. 3. The same player is having an off day; 5 balls go through the goal; the other 95 balls bounce off of the rim. Describe the precision and accuracy of the throws. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 2 Unit 1, Activity 3, Accuracy and Precision Worksheet Answers Figure 1 Figure 2 Figure 3 1. Determine the accuracy and precision represented by each group of darts in the figures above. Explain your choices using complete sentences. Precision? Accuracy? Figure 1 Picture 2 Good All of the darts are grouped in the same area. Poor None of the darts are grouped in the bull’s-eye. Poor None of the darts are grouped in the same area. Poor Few of the darts are grouped in the bull’s-eye. Picture 3 Good All of the darts are grouped in the same area. Good All of the darts are grouped in the bull’s-eye. 2. A basketball player throws 100 free-throws; 95 of these balls go through the goal; 5 miss the goal entirely. Describe the precision and accuracy of the free-throws. The player has good precision and good accuracy because so many of the balls go through the goal. 3. The same player is having an off day; 5 balls go through the goal; the other 95 balls bounce off of the rim. Describe the precision and accuracy of the throws. The player has good precision because so many balls bounce off the rim but poor accuracy because so few balls make it through the goal. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 3 Unit 2, Activity 1, Card Sort Template 1 Matter Homogeneous Pure Substance Heterogeneous Element Mixture Compound Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 4 Unit 2, Activity 1, Card Sort Template 2 Muddy Water Na Solution As salt water Cl Metal NaCl nonmetal Metalloid Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 5 Unit 2, Activity 1, Sample Concept Map Elements, Compounds and Mixtures Concept Map MATTER Can be either MIXTURE PURE SUBSTANCE Is Is ELEMENT Chemically combine to form Can be COMPOUND HOMOGENEOUS HETEROGENEOUS Is either Example METAL Example Na METALLOID Example As NONMETAL Example Cl Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 NaCl Is called SOLUTION Example MUDDY WATER Example SALT WATER Page 6 Unit 2, Activity 2, Sample Word Grid Sample: Homogeneous Can be separated Heterogeneous into individual components The properties of the individual components are the same as properties of the sample Salt Water Copper Salt and water Copper and water Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 7 Unit 2, Activity 2, Sample Word Grid Answers Sample: Salt Water Copper Salt and water Copper and water Homogeneous Can be separated Heterogeneous into individual components X X X X X The properties of the individual components are the same as properties of the sample X X X X X X X Conclusions: 1. Salt (NaCl) is a homogeneous material that can be decomposed into individual elements (sodium and chlorine). The properties of the salt differ from the properties of the elements. Salt is a compound. 2. Water (H2O) is a homogeneous material that can be decomposed into elements (hydrogen and oxygen). Water is a compound. 3. Copper is a homogeneous material that cannot be separated into components. Copper is an element. 4. Salt and water combine to form a homogeneous material that can be separated into parts. When the salt and water are mixed, their properties do not change. Salt water is a homogeneous mixture called a solution. 5. Copper shot and water not homogeneous because the copper and water are easily seen as individual parts. These parts can be separated easily. When the copper and water are mixed, their individual properties do not change. This is a heterogeneous mixture. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 8 Unit 2, Activity 4, Three Worlds of Chemistry Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 9 Unit 2, Activity 5, Density Each box has the same volume. If each ball has the same mass, which box would weigh more? Why? Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 10 Unit 2, Activity 6, Split-Page Notes Physical and Chemical Changes Effervescent tablet in water Observations 1. numerous bubbles formed as soon as tablet touched the water 2. bubbles rose to top of water and burst 3. tablet disappeared 4. bubbles stopped forming 5. looks like nothing else is happening Conclusion The bubbles contained a gas that escaped into the air. The tablet was a solid that underwent a chemical change with the water to produce the gas bubbles. Once the tablet (reactant) was used up, no more gas bubbles (products) were formed, and the reaction stopped. There has been a change in the identity of the material. It is no longer an effervescent tablet. The production of a gas is evidence of a chemical change (reaction) taking place. Cutting a piece of paper Observations 1. smaller pieces of paper are formed Conclusion The smaller pieces of paper are exactly like the original piece of paper (reactant). There has been no change in the identity of the material. It is still paper (product). Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 11 Unit 3, Activity 3, Exploring the Periodic Table 1.01 H 4.00 He 6.94 Li 9.01 Be 10.81 B 12.01 C 14.01 N 16.00 O 19.00 F 20.18 Ne 22.99 Na 24.30 Mg 26.98 Al 28.08 Si 30.97 P 32.07 S 35.45 Cl 39.95 Ar 39.10 K 40.08 Ca 69.72 Ga 72.61 Ge 74.92 As 78.96 Se 79.90 Br 83.80 Kr 85.47 Rb 87.62 Sr 114.82 In 118.71 Sn 121.75 Sb 127.60 Te 126.90 I 131.29 Xe 132.90 Cs 137.33 Ba 204.38 Tl 207.2 Pb 208.98 Bi (209) Po (210) At (222) Rn Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 12 Unit 3, Activity 4, GISTing GISTing The individual Gists are limited to 15 words. Sample paragraph from notes: Atomic radii The atomic radius is ½ the distance between the centers of neighboring atoms. It is the size of the atom due to the size of the electron cloud. Group trends The atomic radii of the main group elements (s & p sublevels) generally increases down a group. The outermost electrons occupy energy levels that are farther from the nucleus. Period trends Atomic radius generally decreases across a period. This is caused by the increasing nuclear charge of the nucleus as you go across a period. More protons are in the nucleus and more electrons are in the same energy level. The increasing nuclear charge attracts the electrons and pulls them closer to the nucleus. Class gist statements for each sentence of the paragraphs 1. Atomic radius means how big an atom is. _____ _____ _____ _____ _____ _____ _____ 2. Atoms get bigger down a group because there are more energy levels. _____ _____ _____ 3. Atoms get smaller across a period because more protons attract the electrons pulling them closer. Summary: Atomic radius (size of the atom) increases down a group because of more energy levels and across a period because of a greater attraction between the larger number of protons and the outer electrons. After several gisting activities, you will be able to construct summaries. Gisting is a mental process and not necessarily a written one. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Unit 4, Activity 1, Vocabulary Self-Awareness Term + - Definition Example Chemical bond Ionic bond Covalent bond Metallic bond Electronegativity Polar covalent bond Nonpolar covalent bond Formula unit Molecule Molecular formula Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 14 Unit 4, Activity 2, Ion Cards Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 15 Unit 4, Activity 2, Ion Cards Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 16 Unit 4, Activity 2, Ion Cards Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 17 Unit 4, Activity 2, Ion Cards Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 18 Unit 4, Activity 3, Chemical Formulas and Nomenclature I Write formulas for the following compounds: 1. copper (I) oxide _______________ 2. aluminum hydroxide _______________ 3. triphosphorus decasulfide _______________ 4. zinc nitrate _______________ 5. hydrobromic acid _______________ 6. mercury (I) bromide _______________ 7. boron tribromide _______________ 8. sodium hydride _______________ 9. barium perchlorate _______________ 10. tetraphosphorus hexasulfide _______________ 11. sulfuric acid _______________ 12. calcium hypochlorite _______________ 13. ammonium phosphite _______________ 14. chromium (III) acetate _______________ 15. hydrosulfic acid _______________ 16. carbonic acid _______________ 17. phosphorus pentafluoride _______________ 18. cobalt (II) nitrate _______________ 19. magnesium sulfate _______________ 20. strontium phosphate _______________ 21. dichlorine monoxide _______________ 22. phosphorous acid _______________ 23. disulfur dichloride _______________ 24. iron (III) carbonate _______________ 25. perchloric acid _______________ Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 19 Unit 4, Activity 3, Chemical Formulas and Nomenclature I Answers Write formulas for the following compounds: 1. copper (I) oxide __Cu2O_______ 2. aluminum hydroxide __Al(OH)3_____ 3. triphosphorus decasulfide __P3S10_______ 4. zinc nitrate __Zn(NO3)2____ 5. hydrobromic acid __HBr(aq)_____ 6. mercury (II) bromide __HgBr2_______ 7. boron tribromide __BBr3________ 8. sodium hydride __NaH________ 9. barium perchlorate __Ba(ClO4)2____ 10. tetraphosphorus hexasulfide __P4S6________ 11. sulfuric acid __H2SO4(aq)___ 12. calcium hypochlorite __Ca(ClO)2____ 13. ammonium phosphite __(NH4)3PO3___ 14. chromium (III) acetate __Cr(C2H3O2)3_ 15. hydrosulfic acid __H2S(aq)_____ 16. carbonic acid __H2CO3(aq)___ 17. phosphorus pentafluoride __PF5_________ 18. cobalt (II) nitrate __Co(NO3)2____ 19. magnesium sulfate __MgSO4______ 20. strontium phosphate __Sr3(PO4)2____ 21. dichlorine monoxide __Cl2O________ 22. phosphorous acid __H3PO4(aq)___ 23. disulfur dichloride __S2Cl2_______ 24. iron (III) carbonate __Fe2(CO3)3___ 25. perchloric acid __HClO4(aq)___ Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 20 Unit 4, Activity 3, Chemical Formulas and Nomenclature II Name the following compounds. 1. K2SO4 ______________________________ 2. N2O4 ______________________________ 3. BaClO4 ______________________________ 4. HNO2(aq) ______________________________ 5. FE2(SO4)3 ______________________________ 6. NH4F ______________________________ 7. BaI2 ______________________________ 8. CrO3 ______________________________ 9. Cu(C2H3O2)2 ______________________________ 10. Ag2CO3 ______________________________ 11. NaOH ______________________________ 12. Ca3(PO4)2 ______________________________ 13. ClF3 ______________________________ 14. K2SO3 ______________________________ 15. AlBr3 ______________________________ 16. MgCl2 ______________________________ 17. HC2H3O2(aq) ______________________________ 18. P2O5 ______________________________ 19. FePO4 ______________________________ 20. SrBr2 ______________________________ 21. Al2S3 ______________________________ 22. LiBr ______________________________ 23. NH3 ______________________________ 24. PbO2 ______________________________ 25. MgO ______________________________ Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 21 Unit 4, Activity 3, Chemical Formulas and Nomenclature II Answers Name the following compounds. 1. K2SO4 __potassium sulfate______________ 2. N2O4 __dinitrogen tetroxide____________ 3. BaClO4 __barium perchlorate_____________ 4. HNO2(aq) __nitrous acid__________________ 5. Fe2(SO4)3 __iron (III) sulfate_______________ 6. NH4F __ammonium fluoride____________ 7. BaI2 __barium iodide_________________ 8. CrO3 __chromium (IV) oxide___________ 9. Cu(C2H3O2)2 __copper (II) acetate_____________ 10. Ag2CO3 __silver carbonate_______________ 11. NaOH __sodium hydroxide______________ 12. Ca3(PO4)2 __calcium phosphate_____________ 13. ClF3 __chlorine trifluoride_____________ 14. K2SO3 __potassium sulfite_______________ 15. AlBr3 __aluminum bromide_____________ 16. MgCl2 __magnesium chloride____________ 17. HC2H3O2(aq) __acetic acid___________________ 18. P2O5 __diphosphorous pentoxide________ 19. FePO4 __iron (III) phosphate____________ 20. SrBr2 __strontium bromide_____________ 21. Al2S3 __aluminum sulfide______________ 22. LiBr __lithium bromide_______________ 23. NH3 __ammonia____________________ 24. PbO2 __lead (IV) oxide_______________ 25. MgO __magnesium oxide_____________ Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 22 Unit 4, Activity 3, Molecular Geometry of Simple Molecules Student Sheet 1 Note: A represents the central atom in the molecule. B represents atoms bonded to the central atom. B can be identical atoms or different atoms. Directions: 1. Find the other students who have the same color balloons as you. Have someone inflate a balloon as much as possible without popping it. Inflate your balloon(s) to the same size. 2. Using the patterns below, tie the appropriate number and color balloons together. For example, for the AB2E model, tie 2 blue balloons and a white balloon together. For groups of 4 balloons, it is easier to tie 2 balloons together and then the other 2 balloons together, then twist the two groups together. For five-balloon groups, make sets of 2 and 3 balloons and twist. For six balloons, use 3 sets of 2 balloons twisted together. 3. Attach a piece of string to hang the finished model from the ceiling. Type of Molecule Balloons Needed for Model One colored balloon models for electron pair geometries AB2 Number of Atoms Attached to the Central Atom 2 2 pink * AB3 3 3 blue * AB2E 3 2 blue, 1 white AB4 4 4 red AB3E 3 3 red, 1 white AB2E2 3 2 red, 2 white AB5 5 5 green AB4E 4 4 green, 1 white AB3E2 3 3 green, 2 white AB2E3 2 2 green, 3 white AB6 6 6 yellow AB5E 5 5 yellow, 1 * * * white AB4E2 4 4 yellow, 2 white Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 23 Unit 4, Activity 3, Molecular Geometry of Simple Molecules Student Sheet 2 Number of lone pairs around the Central Atom Number of atoms attached to the Central Atom Electron Pair Geometry Bond angle of electron pairs Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Type of Molecule Molecular Geometry (Shape of the molecule) Example Page 24 Unit 4, Activity 3, Molecular Geometry of Simple Molecules Answer Sheet Number of lone pairs on the Central Atom 0 Number of atoms attached to the Central Atom 2 0 3 0 4 Tetrahedral 109.5 AB4 1 3 Tetrahedral <109.5 AB3E 2 2 Tetrahedral <109.5 AB2E2 0 5 Trigonal 90, Bipyramidal 120,180 1 4 Trigonal 90, Bipyramidal 120,180 2 3 3 2 0 6 1 2 Electron Pair Geometry Bond angle of Electron pairs Type of Molecule Molecular Shape Example of Molecule Linear 180 AB2 Linear CO2 120 AB3 Trigonal planar AB5 Trigonal planar Tetrahedral Trigonal Pyramidal Bent Trigonal Bipyramidal BF3 CH4 NH3 H2O PCl5 AB4E *See-Saw SF4 90, 180 AB3E2 *T- structure IBr3 180 AB2E3 * Linear XeF2 Octahedral 90, 180 AB6 Octahedral SCl6 5 Octahedral 90, 180 AB5E 4 Octahedral 90, 180 AB4E2 Trigonal Bipyramidal Trigonal Bipyramidal *Pyramidal Planar *Square Planar IF5 XeF4 Note: Molecular Shapes marked * may be omitted if time is a factor. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 25 Unit 4, Activity 4, Chemical Bond Type Lab Purpose: To observe characteristics of ionic and covalent bonds and to classify compounds as ionic or covalent based on those observations. Modified from http://www.hse.k12.in.us/staff/ebutzin/Documents/ICP/Bonding/bond%20types%20lab.doc Safety: Wear goggles. Do not taste or touch any chemicals. Follow guidelines pertaining to an open flame. Materials Test tubes Thin stem pipettes Iron ring and stand Candle with foil holder Small foil pie pan Calcium chloride Citric acid Phenyl salicylate Potassium iodide Sodium chloride Sucrose Conductivity probe Safety goggles Procedure: 1. Place a few crystals of sucrose, sodium chloride, phenyl salicylate, calcium chloride, citric acid and potassium iodide in separate locations around the pie pan as shown in Figure B. Make sure all of the samples are approximately the same size. Do not allow the crystals to touch. Write a brief description of each of the 6 substances in a data table. 2. Testing melting point Place the pie pan on the iron ring. Position the ring so it is just above the tip of a candle flame, as shown in Figure A. Light the candle to check that you have the correct height. Place the candle under the middle of the pan and heat. Record the order in which the substances melt. If a compound doesn’t melt record N/A. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 26 Unit 4, Activity 4, Chemical Bond Type Lab 3. Testing the solubility in water Place a few crystals of each substance in separate test tubes. Add about 1 mL of distilled water and agitate each. Record the solubility in the data table (Yes – if it dissolves, No – if it does not dissolve). 4. Testing the conductivity in water Use the conductivity probe for each of the substances that WERE SOLUBLE in water to determine if they conduct electricity or not. If the compound didn’t dissolve, do NOT try to measure the conductivity. Rinse and dry the probe after each test. Cleanup Rinse all test tubes with water and scrub with a test tube brush. Rinse off the pie pan and scrub with a test tube brush. Dry with a clean cloth. Wash hands and put away goggles. Data Table Compound Description Melting Point (1, 2, 3, 4, N/A) Solubility in Water (Y/N) Conductivity Calcium chloride Citric acid Phenyl salicylate Potassium iodide Sodium chloride Sucrose Write and defend a conclusion based on a logical analysis of your experimental data. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 27 Unit 4, Activity 6, RAFTing R – Role (role of the writer) A – Audience (to whom or what the RAFT is being written) F – Form (the form the writing will take, as in letter, song, etc.) T – Topic (the subject focus of the writing) R – H2O A – Oil F – Letter T – Intermolecular Forces between molecules Dear Oil, I know you would really like for us to get together. Unfortunately, my intermolecular forces are too strong and will always keep us apart. I am a polar molecule. I am attracted to other polar molecules much more than I am attracted to your nonpolar structure. I also have hydrogen bonding which really makes me extremely attractive to other like molecules. I guess you could say that the only thing we really have in common is a really weak dispersion force. Unfortunately, this will not be strong enough for us to base any lasting relationship. Please feel free to look for another molecule with whom to combine. Perhaps you should look for a nonpolar molecule with no tendency to hydrogen bond. Sincerely, Water Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 28 Unit 5, Activity 1, How Large Is a Mole? Materials: 1. 2. 3. 4. Samples of 5 different types of beans Container for measuring the mass of the beans Balance Calculator Procedure: 1. Measure the mass of each type of bean. 2. Using a ratio, students are to calculate the relative masses of the other beans by dividing the mass of the beans by the mass of the smallest bean of the five types used. 3. Count how many whole beans are needed to get the mass in grams equal to the relative mass calculated in step 2 for each type of bean. 4. Using the data in the relative mass column, place the empty container on the balance and zero (tare) the balance. Add beans one at a time to count how many whole beans are needed to get a mass in grams equal to the relative mass for each type of bean. (Mass of container in this example is 25.6g.) Name of bean Mass of the container and the beans (g) Mass of beans (g) Relative mass (g) Number of beans from step 3 Average of Last Column Calculate the average number of whole beans in a container by adding the number of beans in 1 container for each type of bean and dividing by 5. Note: Use the number from the average of the last column box for all calculations. The following ratios can be derived from the data: beans relative mass of beans container container Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 29 Unit 5, Activity 1, How Large Is a Mole? Use the data and the ratios to solve the following problems: 1. Calculate the number of containers given 350 beans of each type of bean. 2. Calculate the number of beans given 5.5 containers of each type of bean. 3. Calculate the mass of 350 containers of each type of bean. 4. Calculate the number of containers given 400 g. of each type of bean. 5. Calculate the number of beans given 400 g of each type of bean. Write and defend a conclusion based on logical analysis of the data obtained from this activity. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 30 Unit 5, Activity 1, How Large Is a Mole? Answer Sheet This activity is designed to help students understand the concept of the mole as a definite number of particles. Using five varieties of different type beans, students will determine the relative mass of each type of bean and express the relative masses in grams. Have students work in groups and provide each group with five sets of 40 beans, a container, and a balance. Have students determine the total mass of each type of bean. Enter the data into the table provided. Using a ratio, students are to calculate the relative masses of the other beans by dividing the mass of the beans by the mass of the smallest bean of the five types used. Using the data in the relative mass column, place an empty container on the balance and zero. Add beans one at a time to count how many whole beans are needed to get a mass in grams equal to the relative mass for each type of beans. (Mass of container in this example is 25.6g.) Name of bean Red beans Large lima beans Chick peas Lentils Black eyes peas Mass of the container and the beans (g) Mass of beans (g) 46.7 21.1 77.9 52.3 44.5 27.7 35.3 18.9 2.1 9.7 Relative mass (g) Number of beans from step 3 211 . 10.0 2.1 52.3 24.9 2.1 9.0 1.0 4.6 Average of last column 19 19 20 19 20 19 Calculate the average number of whole beans in a container. (19 19 20 19 20) 19.4 19 wholebeans 5 Note: Use the number from the average of the last column box for all calculations. The following ratios can be derived from the data: beans relative mass of beans container container Note: Use the average number of beans for the beans/container ratio and the relative mass of each type of bean for the mass/container ratio. Use the data to solve the problems as you would solve mole problems. 1. Calculate the number of containers given 350. beans of each type of bean. 2. Calculate the number of beans given 5.5 containers of each type of bean. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 31 Unit 5, Activity 1, How Large Is a Mole? Answer Sheet 3. Calculate the mass of 350. containers of each type of bean. 4. Calculate the number of containers given 400.g. of each type of bean. 5. Calculate the number of beans given 400.g of each type of bean. Sample Calculations: For these calculations, the container was a cup. 1. 350. beans X 2. 5.5 cups X 1 cup 18.4 cups 19 beans 19 beans 104.5 beans 1 cup 3. 350 red beans x 1cup 21.1g × = 388.7 g 19 red beans 1cup 350 lima beans× 1cup 24.9 g × = 458.68g 19 lima beans 1cup 4. 400. g X 1 cup = 19.0 cups 21.1 g 5. 400. g X 1 cup 19 red beans X = 360.2 red beans 21.1 g 1 cup Write and defend a conclusion based on logical analysis of the data obtained from this activity. Regardless of the type of bean used, the number of beans per cup is consistent (within an acceptable margin of error). Although the mass of each bean is different, the average number of beans per cup is also consistent. The data supports the idea that a cup of beans contains 19 whole beans regardless of the type of bean used or its relative mass. When using this activity as an introduction to mole problems, the container will be used as an analogy to a mole. For example: Calculate the number of containers when given 350 beans of each type of bean. Calculate the number of moles when given 350 atoms of any element. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 32 Unit 5, Activity 3, Observing Chemical Reactions Lab Station 1: Copper wire and silver nitrate solution Materials: small piece of copper wire, pipettes of 0.2 M silver nitrate solution, test tube, test tube rack, waste container Procedure: 1. Record a description of all reactants and products. 2. Make a hook on one end of the copper wire. 3. Hang the wire by the hook in a test tube. 4. Pour enough silver nitrate solution into the tube to cover most of the wire. 5. Place the test tube into the test tube rack and observe for one minute. 6. Record observations. 7. Empty the test tube contents into the large beaker (waste container). Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 33 Unit 5, Activity 3, Observing Chemical Reactions Station 2: zinc + hydrochloric acid Materials: mossy zinc, 0.1M hydrochloric acid, microplate, pipette, waste container 1. Record a description of all reactants and products. 2. Place a piece of mossy zinc a well of a microplate. 3. Add 10 drops of hydrochloric acid to the well. 4. Record observations. 5. Empty the contents of the microplate into the large beaker (waste container). Station 3: sodium chloride + silver nitrate Materials: sodium chloride solution, 0.2 M silver nitrate solution, small test tube, pipette, large beaker 1. Record a description of all reactants and products. 2. Fill a small test tube halfway with sodium chloride solution. 3. Add 3 to 5 drops of the silver nitrate solution. 4. Record observations. 5. Empty the contents of the microplate into the large beaker (waste container). Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 34 Unit 5, Activity 3, Observing Chemical Reactions Station 4: Acetic acid + sodium hydrogen carbonate Materials: Acetic acid (vinegar), sodium hydrogen carbonate (baking soda), 250 ml beaker, waste container 1. Record a description of all reactants and products. 2. Place one teaspoon of sodium hydrogen carbonate into a small beaker. 3. Add three teaspoons of Acetic acid. 4. Record observations. 5. Empty the beaker contents into the large beaker (waste container). Station 5: Acetic acid + sodium hydrogen carbonate + phenolphthalein Materials: Acetic acid (vinegar), sodium hydrogen carbonate (baking soda), phenolphthalein, 250 ml beaker, waste container 1. Record a description of all reactants and products. 2. Place one teaspoon of sodium hydrogen carbonate into a small beaker. 3. Add three teaspoons of Acetic acid. 4. Record observations. 5. Empty the beaker contents into the large beaker (waste container). Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 35 Unit 5, Activity 4, Split-Page Notetaking Patterns for the types of chemical reactions 1. Composition (Synthesis) A + X AX 2. Decomposition AX A + X Single Replacement Reaction: A + BX B + AX or Y + BX X + BY 2 reactants form one product: element + element one compound 2Na + Cl2 2NaCl compound + compound one compound CO2 + H2O H2CO3 one compound two or more products 2NaCl 2Na + Cl2 H2CO3 CO2 + H2O element + compound different element + different compound Use the activity series of the elements to predict the products. Generally elements will replace any element below it on the chart. metals replace less active metals or hydrogen from a compound Cu + AgNO3 Ag + Cu(NO3)2 (reverse reaction will not occur) nonmetals replace less active nonmetals from a compound Double Replacement Reaction: AX + BY AY + BX I2 + NaCl Cl2 + NaI (reverse reaction will not occur) compound + compound different compound + different compound Use a solubility table to predict precipitates (solids) NaCl + AgNO3 NaNO3 + AgCl(s) (reverse reaction will not occur) Neutralization: acid + base → salt + water HCl + NaOH → NaCl + H2O Neutralization is a type of double replacement reaction. An ionic salt is formed from the cation of the base and the anion of the acid. Neutralization is the reaction of the hydronium ions and hydroxide ions to form water molecules. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 36 Unit 5, Activity 6, Can You Make Two Grams? Possible combinations that form precipitates: Reaction Number 1. MgSO4• 7H2O + Ca(C2H3O2)2 • H2O → CaSO4(s) 246.50 g/mol 3.62 g 176.19 g/mol 246.50 g/mol 5.85 g 5.85 g 4.22 g 4.59 g 4.59 g 2.51 g 2.00g 3.37 g 2.99 g → MgCO3(s) + K2SO4 + 7H2O 138.21 g/mol 142.02 g/mol 18.02g/mol 84.31 g/mol 3.28 g 174.27 g/mol 2.00g 176.19 g/mol 3.52g 3.52g + Zn(C2H3O2)2 + 8 H2O 183.48 g/mol 105.99 g/mol 125.38g/mol 1.69g 138.21 g/mol + K2SO4 125.38g/mol 2.20g 138.21 g/mol 18.02 g/mol 2.78g → CaCO3(s) 2.12g 2.01g + 7 H2 O 174.27 g/mol 2.00g 105.99 g/mol + 7 H2O 2.27g ZnCO3(s) 2.12g 142.02 g/mol 18.02g/mol 2.00g → 18.02 g/mol 2.70 g ZnCO3(s) + Na2SO4 8. Ca(C2H3O2)2 • H2O + K2CO3 176.19 g/mol 2.99g 2.00 g → 18.02 g/mol 4.13 136.15 g/mol 2.59g 7. Ca(C2H3O2)2 • H2O + Na2CO3 176.19 g/mol 2.12 g 84.31 g/mol 6. ZnSO4 • 7H2O + K2CO3 287.56 g/mol 2.09 g 105.99 g/mol 5. ZnSO4 • 7H2O + Na2CO3 287.56 g/mol 18.02g + Na2SO4 + 7H2O 4. ZnSO4 • 7H2O + Ca(C2H3O2)2 • H2O → CaSO4(s) 287.56 g/mol 142.38g/mol 2.00 g → MgCO3(s) 3. MgSO4• 7H2O + K2CO3 246.50 g/mol 136.15 g/mol 2.59 g 2. MgSO4• 7H2O + Na2CO3 + Mg(C2H3O2)2 + 7H2O 2.01g + 2NaC2H3O2 + H2O 100.09 g/mol 82.03 g/mol 18.02 g/mol 2.00g 3.28g → CaCO3(s) + 2KC2H3O2 100.09 g/mol 98.15 g/mol 2.76g Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 2.00g 3.92g 036g + H2 O 18.02g/mol 0.36g Page 37 Unit 5, Activity 7, Vocabulary Self-Awareness Term + - Definition Example Oxidation Reduction Redox reaction Oxidizing agent Reducing agent Skeleton equation Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 38 Unit 5, Activity 7, Introduction to Oxidation-Reduction Reactions Half- fill each well in the column with the indicated solution. Column 1: Zn(NO3)2 Column 2: Pb(NO3)2 Column 3: Cu(NO3)2 1. Place one piece of zinc shot in each filled well in row 1. 2. Place one piece of lead shot in each filled well in row 2. 3. Place one piece of copper shot in each filled well in row 3. Column 1 2 3 Row 1 2 3 4. Watch for two minutes, then record observations. 5. Make a list of the elements in order of reactivity. 6. Write the redox equation for each chemical change. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 39 Unit 5, Activity 7, Introduction to Oxidation-Reduction Reactions Answer Sheet 1. Half- fill each well in the column with the indicated solution. Column 1: Zn(NO3)2 Column 2: Pb(NO3)2 Column 3: Cu(NO3)2 2. Place one piece of zinc shot in each filled well in row 1. 3. Place one piece of lead shot in each filled well in row 2. 4. Place one piece of copper shot in each filled well in row 3. Column 1 2 3 Row 1 2 3 5. Watch for two minutes, then record observations. 6. Make a list of the elements in order of reactivity. 7. Write the redox equation for each chemical change. 8. Write a conclusion based on your observations. Elements in order of reactivity: Zn, Pb, Cu LEO the lion says GER (loss of electrons is oxidation, gaining electrons is reduction) Redox equations: Zn + Pb(NO3)2 → Pb + Zn(NO3)2 Zn0 → Zn 2+ + 2e- (oxidation) Pb 2+ + 2e- → Pb0 (reduction) Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 40 Unit 5, Activity 7, Introduction to Oxidation-Reduction Reactions Answer Sheet Zn + Cu(NO3)2 → Cu + Zn(NO3)2 Zn0 → + Zn 2+ 2e- (oxidation) Cu2++ 2e- → Cu0 (reduction) Pb + Zn(NO3)2 → NR (no reaction) Pb + Cu(NO3)2 → Cu + Pb(NO3)2 Pb0 → Pb2+ +2e- (oxidation) Cu2++2e- → Cu0 (reduction) Cu + Zn(NO3)2 → NR Cu + Pb(NO3)2 → NR Conclusion: Oxidation is the process by which electrons are removed from atoms or ions. Zn is oxidized by the other two ions. Pb is only oxidized by the Cu2+ ion. Cu is not oxidized by either ion. Zn gives up its electrons more easily than the other ions.The element that is oxidized is the reducing agent therefore Zn is the strongest reducing agent, followed by Pb and lastly, by Cu. Reduction is the process by which electrons are added to atoms or ions. The element that is reduced is the oxidizing agent. Cu is the strongest oxidizing agent, followed by Pb and then Zn. Oxidation and reduction must take place at the same time because the number of electrons lost must equal the number of electrons gained. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 41 Unit 5, Activity 7, Introduction to Oxidation-Reduction Reactions Answer Sheet Answers to ionic equations: Molecular equation: Zn + Pb(NO3)2 → Pb + Zn(NO3)2 Ionic Equation: Zn0 + Pb2+ + 2NO3-1 → Pb0 + Zn 2+ + 2NO3-1 Net Ionic Equation: Zn0 + Pb 2+→ Pb0 + Zn 2+ Molecular equation: Zn + Cu(NO3)2 → Cu + Zn(NO3)2 Ionic equation: Zn0 + Cu2+ + 2NO3-1 → Cu0+ Zn 2+ + 2NO3-1 Net Ionic equation: Zn0 + Cu2+ → Cu0+ Zn 2+ Molecular equation: Pb + Zn(NO3)2 → NR (no reaction) Molecular equation: Pb + Cu(NO3)2 → Cu + Pb(NO3)2 Ionic equation: Pb0 + Cu2+ +2NO3-1 → Cu0 + Pb2+ + 2NO3-1 Net Ionic equation: Pb0 + Cu2+ → Cu0 + Pb2+ Molecular equation: Cu + Zn(NO3)2 → NR Molecular equation: Cu + Pb(NO3)2 → NR Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 42 Unit 6, Activity 4, Heating Curve Gas State Cp (g) Boiling Point (Hv) Liquid State Cp (l) Melting Point (Hf) Solid State Cp(s) Heat is added to a substance in the solid state. The energy added will increase the temperature of the substance to its specific melting point. The amount of energy required to raise the temperature depends on the specific heat (Cp) and the state of the substance. Specific heat is the amount of energy needed to raise the temperature of one gram of a substance by one degree Celsius. At the melting point, the temperature stops rising and the substance starts to melt. The energy supplied is used to weaken the intermolecular forces of attraction and the temperature remains constant. The amount of energy needed to melt a substance depends on its heat of fusion (H f). The molar heat of fusion is the amount of energy required to melt one mole of a substance at its melting point. After the phase change is complete, the temperature rise will follow a different rate than that of the solid because the liquid state has a different heat capacity. At the boiling point, the temperature stops rising and the substance starts to boil. The energy supplied is used to break the intermolecular forces of attraction and the temperature remains constant. The amount of energy needed to boil a substance depends on its heat of vaporization (Hv). The molar heat of vaporization is the amount of energy required to boil one mole of a substance at its boiling point. The Hv is higher than the Hf because of breaking the forces of attraction. After the phase change is complete, the temperature rise will follow a different rate than that of the liquid because the gaseous state has a different heat capacity. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 43 Unit 6, Activity 4, Phase Diagrams A phase diagram is a graph of the conditions of temperature and pressure at which the solid, liquid, and gaseous phases of a substance exist. The lines separating the phases are called phase boundaries. Each point on the phase boundary show the conditions under which the two phases exist in dynamic equilibrium. Each point along the solid/liquid phase boundary represents the temperature and pressure combinations where the rate of the solid melting is equal to the rate of the liquid freezing. Each point represents a melting point. Each point along the liquid/ vapor phase boundary represents the temperature and pressure combinations where the rate of the liquid boiling is equal to the rate of the vapor condensing. Each point represents a boiling point. Each point along the solid/gas phase boundary represents the temperature and pressure combinations where the rate of the solid subliming is equal to the rate of the vapor condensing to a solid (called deposition). Each point represents a sublimation point. The triple point indicates the only temperature and pressure conditions where the solid, liquid, and vapor phases are all in equilibrium. The critical point (Pc) is the point above which a substance will always be a gas regardless of the pressure and temperature. The critical temperature is the highest temperature a substance can exist as a liquid. The critical pressure is the lowest pressure required for the substance to be a liquid at the critical temperature. Phase Diagram for H2O Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Phase diagram for CO2 Page 44 Unit 6, Activity 6, Exothermic and Endothermic Energy Diagrams Heat of reaction (H) is the amount of heat released or absorbed during a chemical reaction. H = H products - H reactants The heat content of the reactants is higher than the heat content of the products. Energy in the form of heat will be released when the products form. The heat of reaction (H) is negative. Example: 2 H2(g) + O2(g) → 2H2O(g) H = - 483.6 kJ The heat content of the reactants is lower than the heat content of the products. Energy in the form of heat must be absorbed (added) to form the products. The heat of reaction (H) is positive. Example: 2 H2O(g) → 2H2(g) + O2(g) H = + 483.6 kJ Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 45 Unit 6, Activity 6, Energy Diagram (with activation energy) For a reversible reaction, the activated complex is the same. The activated complex occurs at the maximum-energy position along the reaction pathway. The activation energy of the forward reaction is lower than the activation energy of the reverse reaction in this energy diagram. The H is the same amount for both reactions but the sign of H is negative for the forward reaction and is positive for the reverse reaction. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 46 Unit 7, Activity 1, Vocabulary Self- Awareness Term + - Definition Example Solution Solute Solvent Soluble Electrolyte Nonelectrolyte Colloid Solubility Saturated solution Unsaturated solution Supersaturated Solution equilibrium Miscible Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 47 Unit 7, Activity 1, Vocabulary Self- Awareness Immiscible chromatography molarity molality Colligative property Vapor pressure Nonvolatile Volatile Freezing-point depression Boiling-point elevation Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 48 Unit 7, Activity 3, Solution Concentrations Sample Problems using factor-label method. Molarity (M) = moles of solute liter of solution Example 1: Calculate the molarity of a 1500 ml solution that contains 45.0 g of MgCl2. M= 45.0 g MgCl 2 1 mol MgCl 2 1000 mL X X 0.31 M 1500 mL of solution 95.3 g MgCl 2 1L Example 2: Calculate the mass of solute in 750.0 mL of a 0.500 M H2SO4 solution. Mass of solute = 750 mL X 1L 0.500mol H 2SO 4 98.1g H 2SO 4 X X 36.8g H 2SO 4 1000 mL 1L 1mol H 2SO 4 Example 3: Calculate the volume of solution that can be made using a 6.00 M solution using 45.0 g C6H12O6. Volume = 45.0g C6 H12 O 6 X 1mol C6 H12 O 6 1L X 0.400 L 180.0g C6 H 12 O 6 6.00 mol C6 H 12 O 6 Molality (m) = moles of solute kg of solution Example 1: Calculate the molality of a solution containing 50.0 g of HC2H3O2 dissolved in 500.0 g Hm=O. m= 50.0g HC 2 H 3O 2 1mol HC 2 H 3O 2 1000g H 2O X X =1.67 m 500.0g H 2O 60.0gHC 2 H 3O 2 1kg H 2O Example 2: Calculate the mass of solute needed to make a 0.450 m NaOH solution containing 750.0 g H2O. mass solute = 750.0g H 2O X 1kg 0.450 mol NaOH 40.0 g NaOH X X =13.5g NaOH 1000g 1kg 1mol NaOH Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 49 Unit 7, Activity 3, Solution Concentrations Example 3: Calculate the mass of solvent needed to make a 2.50 m H2SO4 solution containing 150.0 g of the acid. mass H2O = 150.0g H 2SO 4 X 1mol H 2SO 4 1kg H 2O 1000g X X = 612 g H 2O 98.1g H 2SO 4 2.50 mol H 2SO 4 1kg Mole Fraction (X) = moles of solute (or solvent) moles of solute + solvent Example: Determine the mole fraction of glucose, C6H12O6, in a solution containing 425 g glucose dissolved in 750.0 g H2O. Moles of glucose: 425g C6 H12 O6 X 1mol C6 H12O6 = 2.36 mol C6 H12O6 180g C6 H12 O6 Moles of water: 750.0 g H 2O X 1mol H 2O = 41.7 mol H 2O 18.0 g H 2 O Total moles of solute + solvent: 2.36 mol + 41.7 mol= 44.06 mol X molglu cos e Mole fraction of C6H12O6: XC6H12O6 = molC6H12O6 mol C6H12O6 +H2O = 2.36 mol = 0.5 44.06 mol Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 50 Unit 7, Activity 5, Ice Cream Recipe Recipe for 40 pint size bags of ice cream (1 bag per student): 1 gal whole milk 1 pint half & half 6 cups sugar 6 t vanilla Additional materials needed: spoon, large pot, several boxes of ice cream salt; two rolls of duct tape, several large bags of ice, enough newspaper for each student to have a section, plastic bags from grocery or discount stores Combine all ingredients in the pot to make the ice cream mixture and heat until the sugar is dissolved. Stir the mixture often to prevent the mixture from scorching. Pour into the empty milk container. There may some extra mixture, so have a smaller container or zip top bag handy also. Per student: 1 pint size freezer zip top bags 1 gallon size freezer bags 2 plastic bags a section of newspaper ice cream salt ice duct tape Directions: Fill the gallon bag half full of ice. Add 1/2 inch layer of ice cream salt. Put 1/2 cup of ice cream mixture in small bag. Seal the small bag and place duct tape over the sealed end. Put the small bag inside of large one. Add enough ice to fill the gallon bag. Seal and duct tape the sealed end of the large bag. Wrap the large bag with several layers of newspaper. Place the wrapped bag in a couple of plastic bags. Tie the ends of the plastic bags. Shake or rotate the bags gently for about 15 min. Have a large container such as a dish pan handy to empty the water/ salt mixture into. The water can be evaporated and the salt reused, if desired. Powdered drink mixes can be made according to the directions on the package and used in place of the ice cream. A “slush” will be formed. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 51 Unit 7, Activity7, pH Lab Carousels Lab Carousel 1: At each station place: a microplate, red litmus paper, blue litmus paper, universal indicator and phenolphthalein (and any other available indicators), and stirring rod. Pipettes containing: Station 1: vinegar Station 4: household ammonia solution Station 2: distilled water Station 5: colorless soda Station 3: KOH solution Station 6: HCl solution Instruct students to test each solution with the indicator papers and indicators at each station and identify each of the solutions as acids or bases. Data should be recorded in a student –generated data table. Lab Carousel 2: At each station place a microplate, pH paper and/ or pH meter, and stirring rod. Pipettes containing: Station 1: vinegar Station 4: household ammonia solution Station 2: distilled water Station 5: colorless soda Station 3: KOH solution Station 6: HCl solution Instruct students to determine the pH of the solutions and rank them in order of increasing pH. Data should be recorded in a student –generated data table. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 52 Unit 8, Activity 2, Alkanes Alkanes are saturated hydrocarbons (compounds containing only carbon and hydrogen) with the formula CnH2n+2, where “n” represents the number of carbon atoms. “Saturated” means that all C-C bonds are single bonds. Names of organic compounds follow the rules of IUPAC (International Union of Pure and Applied Chemistry). Notice that each compound differs from the previous one by a –CH2 group. A homologous series in one in which the compounds differ from each other by a specific unit. The pattern for the first 10 alkanes is shown below. Stem name Alkane name Formula meth- methane CH4 Number of isomers 1 eth- ethane C2H6 1 prop- propane C3H8 1 but- butane C4H10 2 pent- pentane C5H12 3 hex- hexane C6H14 5 hept- heptane C7H16 9 oct- octane C8H18 18 non- nonane C9H20 35 dec- decane C10H22 75 Isomers are compounds with the same molecular formula but different structural formulas. Draw the isomers for pentane and hexane. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 53 Unit 8, Activity 2, Alkanes Rules for naming alkanes: 1. Pick out the longest continuous chain of carbon atoms and name it. 2. Number the carbon atoms from the end that will give the lowest numbers possible to the branches. 3. Name the branches by adding –yl to the stem name and adding a number to indicate the carbon atom the branch is attached to. The number will be followed by a dash. All branches must have a number with it. Numbers are separated by commas. *If branches are different groups, they appear alphabetically in the name. 4. If more than one of an alkyl group appears, a number prefix is used to denote the total number of groups. 5. Dashes between carbon atoms do not need to be shown. Examples: 6 5 4 3 CH3 CH2 CH2 CH CH3 2 CH2 1 CH3 Name: 3-methylhexane CH3 CH3 CH3CHCHCH2CHCH2 CH3 CH2 CH3 Name: 3-ethyl-2,5-dimethylheptane 1 2 3 4 5 CH3 CH CH2 CH CH3 CH3 CH3 Name: 2,4-dimethylpentane CH3 CH2 CH3CHCHCH3 CHCH2CH3 CH3 Name: 3,4,5-trimethylheptane Name the isomers for pentane and hexane that were drawn on the previous sheet. Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 54 Unit 8, Activity 2, Alkanes Answer Sheet Isomers of Pentane (formula C5H12): 1. CH3CH2CH2CH2CH3 n-pentane ( n means normal straight chain) 2. CH3CHCH2CH3 2- methylbutane CH3 3. CH3 2,2-dimethylpropane CH3CCH3 CH3 Isomers of hexane (formula C6H14) 1. CH3CH2CH2CH2CH2CH3 n-hexane 2. CH3CHCH2CH2CH3 2-methylpentane CH3 3. CH3CH2CHCH2CH3 3-methylpentane CH3 4. CH3 CH CH CH3 2,3-dimethylbutane CH3 CH3 CH3 5. CH3 C CH2 CH3 2.3-dimethylbutane CH3 Blackline Masters, Chemistry Louisiana Comprehensive Curriculum, Revised 2008 Page 55