* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download cofactorsA
Survey
Document related concepts
Photosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biosynthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
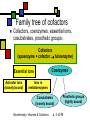
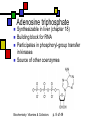
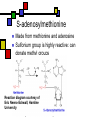
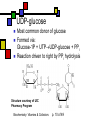
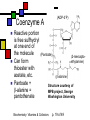
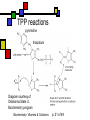
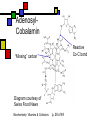
Many cofactors are derived from vitamins We justify lumping these two topics together because many cofactors are vitamins or are metabolites of vitamins. Biochemistry: Vitamins & Cofactors p. 1 of 49 What we’ll discuss Tightly-bound metal ions as cofactors Activator ions as cofactors Cosubstrates Prosthetic groups Biochemistry: Vitamins & Cofactors Vitamins Water-soluble vitamins Ascorbate Cofactors Fat-soluble vitamins p. 2 of 49 Family tree of cofactors Cofactors, coenzymes, essential ions, cosubstrates, prosthetic groups: Cofactors (apoenzyme + cofactor holoenzyme) Coenzymes Essential ions Activator ions (loosely bound) Ions in metalloenzymes Cosubstrates (loosely bound) Biochemistry: Vitamins & Cofactors Prosthetic groups (tightly bound) p. 3 of 49 Metal-activated enzymes Absolute requirements for mobile ions Often require K+, Ca2+, Mg2+ Example: Kinases: Mg-ATP complex Metalloenzymes: firmly bound metal ions in active site Usually divalent or more Sometimes 1e- redox changes in metal Biochemistry: Vitamins & Cofactors p. 4 of 49 Coenzymes Organic moeities that enable enzymes to perform their function: they supply functionalities not available from amino acid side chains Cosubstrates Enter reaction, get altered, leave Repeated recycling within cell or organelle Prosthetic groups Remain bound to enzyme throughout Change during one phase of reaction, eventually get restored to starting state Biochemistry: Vitamins & Cofactors p. 5 of 49 Major cosubstrates Facilitate group transfers, mostly small groups Oxidation-reduction participants Cosubstrate ATP S-adenosylMet UDP-glucose NAD,NADP Coenzyme A Tetrahydrofolate Ubiquinone Source Function Transfer P,Nucleotide Methyl transfer Glycosyl transfer Niacin 2-electron redox Pantothenate Acyl transfer Folate 1Carbon transfer Lipid-soluble e- carrier Biochemistry: Vitamins & Cofactors p. 6 of 49 Major prosthetic groups Transfer of larger groups One- or two-electron redox changes Prosth.gp. FMN, FAD TPP PLP Biotin Adenosylcobalamin MeCobal. Lipoamide Retinal Vitamin K Source Riboflavin Thiamine Pyridoxine Biotin Cobalamin Function 1e- and 2e- redox transfers 2-Carbon transfers with C=O Amino acid group transfers Carboxylation, COO- transfer Intramolec. rearrangements Cobalamin Methyl-group transfers Transfer from TPP Vision Carboxylation of glu residues Vitamin A Vitamin K Biochemistry: Vitamins & Cofactors p. 7 of 49 Adenosine triphosphate Synthesizable in liver (chapter 18) Building block for RNA Participates in phosphoryl-group transfer in kinases Source of other coenzymes Biochemistry: Vitamins & Cofactors p. 8 of 49 S-adenosylmethionine Made from methionine and adenosine Sulfonium group is highly reactive: can donate methyl groups Reaction diagram courtesy of Eric Neeno-Eckwall, Hamline University Biochemistry: Vitamins & Cofactors p. 9 of 49 UDP-glucose Most common donor of glucose Formed via: Glucose-1P + UTPUDP-glucose + PPi Reaction driven to right by PPi hydrolysis Structure courtesy of UIC Pharmacy Program Biochemistry: Vitamins & Cofactors p. 10 of 49 NAD+ and NADP+ Net charge isn’t really >0 ; the + is just a reminder that the nicotinamide ring is positively charged Most important cosubstrates in oxidationreduction reactions in aerobic organisms Structure courtesy of Sergio Marchesini, U. Brescia Biochemistry: Vitamins & Cofactors p. 11 of 49 Differences between them The chemical difference is in the phosphorylation of the 2’ phosphate group of the ribose moiety The functional difference is that NAD is usually associated with catabolic reactions and NADP is usually associated with anabolic reactions Therefore often NAD+ and NADPH are reactants and NADH and NADP+ are products Biochemistry: Vitamins & Cofactors p. 12 of 49 How do we get back to the starting point? NADH is often oxidized back to NAD+ as part of the electron-transport chain Imbalances can be addressed via NAD Kinase (S.Kawai et al (2005), J.Biol.Chem. 280:39200) and NADP phosphatase Biochemistry: Vitamins & Cofactors p. 13 of 49 iClicker quiz: single question What would you expect to be the phosphate donor in the NAD kinase reaction? (a) free phosphate (b) pyrophosphate (c) ATP (d) pyridoxal phosphate Biochemistry: Vitamins & Cofactors p. 14 of 49 Reduced forms of NAD(P) Reduction occurs on the nicotinamide ring Ring is no longer netpositive Ring is still planar but the two hydrogens on the para carbon are not Biochemistry: Vitamins & Cofactors p. 15 of 49 FAD and FMN Flavin group based on riboflavin Alternate participants in redox reactions Prosthetic groups: tightly but noncovalently bound to their enzymes That protects against wasteful reoxidation of reduced forms FADH2 is weaker reducing agent than NADH These are capable of one-electron oxidations and reductions Biochemistry: Vitamins & Cofactors p. 16 of 49 FAD and FMN structures FAD has an AMP attached P to P Structure courtesy Paisley University Biochemistry: Vitamins & Cofactors p. 17 of 49 FMN/FAD redox forms Two-electron version: H+ + :H- transferred Reaction diagram courtesy of Eric Neeno-Eckwall, Hamline University Biochemistry: Vitamins & Cofactors p. 18 of 49 (ADP-3’P) Coenzyme A Reactive portion is free sulfhydryl at one end of the molecule Can form thioester with acetate, etc. Pantoate + b-alanine = pantothenate (Pantoate) 2-mercaptoethylamine) b-alanine) Structure courtesy of MPB project, George Washington University Biochemistry: Vitamins & Cofactors p. 19 of 49 Thiamine Pyrophosphate Based on thiamine, vitamin B1 Carboxylases and oxidative decarboxylases use this coenzyme So do transketolases (move 2 carbons at a time between sugars with keto groups) Thiazolium ring is reactive center: pKa drops from 15 in H2O to 6 in enzyme Biochemistry: Vitamins & Cofactors p. 20 of 49 TPP reactions pyrimidine thiazolium Diagram courtesy of Oklahoma State U. Biochemistry program Biochemistry: Vitamins & Cofactors p. 21 of 49 Pyridoxal phosphate PLP is prosthetic group for many amino-acid-related enzymes, particularly transaminations Carbonyl group of PLP bound as a Schiff base (imine) to -amino group of lysine at active site First step is always formation of external aldimine; goes through gem-diamine intermediate to internal aldimine Biochemistry: Vitamins & Cofactors p. 22 of 49 Biotin Rarity: vitamin is the prosthetic group Used in reactions that transfer carboxyl groups … and in ATP-dependent carboxylations Biochemistry: Vitamins & Cofactors p. 23 of 49 Biotin reactivity Covalently bound to active-site lysines to form species called biocytin Pyruvate carboxylase is characteristic reaction: Diagram courtesy University of Virginia Biochemistry Biochemistry: Vitamins & Cofactors p. 24 of 49 Tetrahydrofolate Primary donor of one-carbon units (formyl, methylene, methyl) Supplies methyl group for thymidylate Dihydrofolate reductase (DHFR) is an interesting drug target Methotrexate as cancer chemotherapeutic: cancer needs more thymidylate than healthy cells Trimethoprim as antibacterial: Bacterial DHFR is somewhat different from eucaryotic DHFR because bacteria derive DHF from other sources; humans get it from folate Biochemistry: Vitamins & Cofactors p. 25 of 49 THF structure and function Figure courtesy horticulture program, Purdue Biochemistry: Vitamins & Cofactors p. 26 of 49 Cobalamin Largest B vitamin Structure related to heme but missing one carbon in ring structure Cobalt bound in core of ring system Involved in enzymatic rearrangements Catabolism of odd-chain fatty acids Methylation of homocysteine Reductive dehalogenation Biochemistry: Vitamins & Cofactors p. 27 of 49 AdenosylCobalamin Reactive Co-C bond “Missing” carbon Diagram courtesy of Swiss Food News Biochemistry: Vitamins & Cofactors p. 28 of 49 Lipoamide Protein-bound form of lipoic acid Contains five-membered disulfide ring Covalently bound via amide to protein lysine sidechain Involved in swinging arm between active sites in multienzyme complexes Disulfides break periodically Example: pyruvate dehydrogenase complex Biochemistry: Vitamins & Cofactors p. 29 of 49 Lipoamide 2e- reduction Cf. Scheme 7.6: thioester starting point Fig. Courtesy Biochem and Biophysics program, Rensselaer Biochemistry: Vitamins & Cofactors p. 30 of 49 iClicker revisited Which coenzyme would you expect would be required for the reaction oxaloacetate + glutamate aspartate + a-ketoglutarate? (a) ascorbate (b) PLP ( c) thiamine pyrophosphate (d) NAD (e) none of the above Biochemistry: Vitamins & Cofactors p. 31 of 49 Vitamins: necessary micronutrients that cannot be synthesized internally What’s a vitamin for one organism is not for another Primates and some rodents are the only vertebrates that don’t synthesize ascorbate E.coli can make almost everything given energy and sources of atoms Biochemistry: Vitamins & Cofactors p. 32 of 49 Why wouldn’t organisms make everything? Complex metabolites require energy for synthesis Control of their synthesis is also metabolically expensive Cheaper in the long run to derive these nutrients from diet Biochemistry: Vitamins & Cofactors p. 33 of 49 Vitamins: broad classifications Water-soluble vitamins Coenzymes or coenzyme precursors Non-coenzymic metabolites Fat-soluble vitamins Antioxidants Other lipidic vitamins Biochemistry: Vitamins & Cofactors p. 34 of 49