* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download HIV Pharmacotherapy Focused Update
Survey
Document related concepts
Psychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
HIV vaccine wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Tablet (pharmacy) wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Transcript
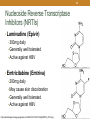
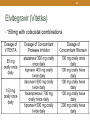
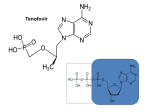
1 HIV Pharmacotherapy Focused Update Drew Lambert, PharmD [email protected] Husson University School of Pharmacy PollEverywhere - Text DREWLAMBERT221 to 22333 to join You can respond at PollEv.com/drewlambert221 2 I have no conflicts of interest. • However, I will be using brand names extensively during the presentation PollEverywhere - Text DREWLAMBERT221 to 22333 to join You can respond at PollEv.com/drewlambert221 3 Please register for PollEverywhere • You can respond at PollEv.com/drewlambert221 • Text DREWLAMBERT221 to 22333 once to join • Standard text messaging rates apply • No additional charges 4 5 6 10 Objectives • Identify common antiretroviral drugs • Review most recent HIV guidelines • Choose appropriate antiretroviral regimens PollEverywhere - Text DREWLAMBERT221 to 22333 to join You can respond at PollEv.com/drewlambert221 11 Definitions • HIV – Human Immunodeficiency Virus • AIDS – Acquired Immune Deficiency Syndrome • ARV – Antiretroviral • ART – Antiretroviral Therapy • HAART – Highly Active Antiretroviral Therapy • NRTI – Nucleoside Reverse Transcriptase Inhibitor • NNRTI – Non-nucleoside Reverse Transcriptase Inhibitor • PI – Protease Inhibitor • INSTI – Integrase Strand Transfer Inhibitor 12 Common ARV Drugs (part of 1st line regimens) For a list of all FDA approved HIV drugs: https://aidsinfo.nih.gov/education-materials/factsheets/19/58/fda-approved-hiv-medicines 13 Abacavir (Ziagen) • Nucleoside reverse transcriptase inhibitor (NRTI) • 600mg once daily or 300mg BID • Must test for HLA-B*5701 because of possible hypersensitivity reaction • Nausea, headache, malaise and fatigue, nausea and vomiting, and dreams/sleep disorders 14 Nucleoside Reverse Transcriptase Inhibitors (NRTIs) • Lamivudine (Epivir) • 300mg daily • Generally well tolerated • Active against HBV • Emtricitabine (Emtriva) • 200mg daily • May cause skin discoloration • Generally well tolerated • Active against HBV http://patentimages.storage.googleapis.com/WO2012137227A2/imgf000012_0001.png 15 Tenofovir disoproxil fumarate (Viread, TDF) • NucleoTIDE reverse transcriptase inhibitor (NRTI) • 300mg daily • Possible decreases in BMD • Well tolerated • Activity against HBV • May cause renal dysfunction • Dose adjustments needed for CrCL <50mL/min, <30mL/min, and is not recommended with CrCl <10 unless receiving hemodialysis 16 Tenofovir alafenamide (no brand, TAF) • NucleoTIDE reverse transcriptase inhibitor (NRTI) • 25mg or 10mg if given with cobicistat • Only available in combination with other drugs • Both as a dual NRTI combination and single tablet regimen combinations • Well tolerated • May use down to CrCl of 30mL/min 17 Tenofovir disoproxil fumarate (TDF) vs. Tenofovir alafenamide (TAF) • TDF conversion to tenofovir occurs mainly in the plasma; TAF conversion occurs intracellularly • Plasma levels 91% lower; intracellular levels 4.1x higher • Less serum creatinine increase • Less effects on bone mineral density (BMD) • Less proteinuria • Less renal dysfunction • Same price • More comparison studies are ongoing Genvoya – A New 4-Drug Combination for HIV. The Medical Letter. 2016;15(1488):19-21. 18 Darunavir (Prezista) • Protease inhibitor (PI) • 800mg daily boosted with ritonavir for treatment naïve patients, 600mg BID boosted for treatment experienced • Take with food • Very high barrier to resistance • Not recommended in severe liver disease • Less metabolic side effects than older PIs • Possible rash on initiation 19 Ritonavir (Norvir) • Protease inhibitor (PI) • Pharmacoinetic booster—100mg with each dose of the other drug • Available as tablets and capsules—tablets much more palatable • Tingling or numbness of the hands or feet, or around the mouth • Nausea/vomiting 20 Cobicistat (Tybost, cobi) • 150mg daily • Pharmacokinetic booster (3A4 inhibitor) approved to be used in combination with • Darunavir 800mg daily (Prezcobix) • Atazanavir 300mg daily (Evotaz) • Elvitegravir 150mg as part of Stribild or Genvoya • Not active against HIV • Inhibits creatinine excretion but does not change GFR 21 Raltegravir (Isentress) • Integrase strand transfer inhibitor (INSTI) • 400mg BID • No food requirements • No renal dose adjustments • Not studied in severe hepatic impairment • Metabolized by UGT1A1 mediated glucuronidation • 800mg twice daily with rifampin • Increased total bilirubin • Elevated CK – myopathy and rhabdomyolysis (rare) 22 Elvitegravir (Vitetka) • Integrase strand transfer inhibitor (INSTI) • Must be given with ritonavir boosted protease inhibitors • Take with food • Diarrhea is the most common adverse event • Avoid with CYP 3A4 inducers • Dosing based on what it is administered with 23 Elvitegravir (Vitetka) • 150mg with cobicistat combinations 24 Dolutegravir (Tivicay) • Integrase strand transfer inhibitor (INSTI) • 50mg daily • Increase to 50mg twice daily when given with UGT1A1 inducers (e.g., rifampin, efavirenz, fosamprenavir, tipranavir) or with INSTI resistance • Headache, insomnia, fatigue • Take 2 hours prior or 6 hours after antacids • No food effects 25 COMBINATION TABLETS Not all inclusive 26 Epzicom • Lamivudine 300mg and abacavir 600mg • 1 tablet daily • Must test patients for HLA*B-5701 allele • Do not use if CrCl <50mL/min • Diarrhea, nausea, and headache • No food requirement 27 Truvada • Emtricitabine 200mg and tenofovir disoproxil fumarate 300mg • Dual NRTI combination • 1 tablet daily with normal renal function • CrCl 30-49mL/min – 1 tablet q48h • Nausea and diarrhea • No food requirement 28 Descovy – April 2016 • Emtricitabine 200mg + tenofovir alafenamide 25mg • 1 tablet daily • Dual NRTI combination • Similar to Truvada • CrCl 30mL/min or greater • Nausea is most common ADR • No food requirement 29 SINGLE TABLET REGIMENS All inclusive (at this point in time) All are 1 tablet daily 30 Atripla • Efavirenz 600mg + emtricitabine 200mg + tenofovir disoproxil fumarate 300mg • NNRTI based regimen • Do not use with CrCl <50mL/min • Dizziness, vivid dreams or nightmares, rash, nausea/vomiting/diarrhea • Usually taken at bedtime on an empty stomach 31 Complera • Rilpivirine 25mg + emtricitabine 200mg + tenofovir disoproxil fumarate 300mg • NNRTI based regimen • Administer with food • Do not use with CrCl <50mL/min • Depression, insomnia, headache, nausea, vomiting 32 Odefsey – March 2016 • Rilpivirine 25mg + emtricitabine 200mg + tenofovir alafenamide 25mg • NNRTI based single tablet regimen • Similar to Complera • Do not use with CrCl <30mL/min • Take with food • Depression, insomnia, headache, nausea are common 33 Stribild • Elvitegravir 150mg + cobicistat 150mg + emtricitabine 200mg + tenofovir disoproxil fumarate 300mg • INSTI based single tablet regimen • Do start in patients with CrCl <70mL/min and do not use with CrCl <50mL/min • Common adverse events • Nausea and diarrhea • Take with food • Take antacids 2 hours before or after Stribild 34 Genvoya – November 2015 • Elvitegravir 150mg + cobicistat 150mg + emtricitabine 200mg + tenofovir alafenamide 10mg • INSTI based single tablet regimen • Similar to Stribild • Do not use with CrCl <30mL/min • Nausea is most common ADR • Take with food 35 Triumeq • Dolutegravir 50mg + abacavir 600mg + lamivudine 300mg • Integrase inhibitor based single tablet regimen • Do not use with CrCl <50mL/min • 2nd generation INSTI • Only combination with abacavir/lamivudine NRTI backbone 36 MATCHING EXERCISE 37 HIV TREATMENT GUIDELINES 38 • Who should be treated? • Everyone with HIV • When should treatment be started? • As soon as possible • How long do we treat for? • Forever 39 What do we treat with? A usual regimen contains: • 2 NRTIs • 1 drug from a different class • INSTI • PI • NNRTI • +/- Pharmacokinetic booster • Ritonavir (Norvir) • Cobicistat (Tybost) 40 How do we monitor patients? • Viral Load = Amount of virus per mL of blood • Goal: As low as possible! • <50 copies/mL correlates with durable response to HIV medications and is considered “undetectable” • Newer assays may detect < 20 copies/mL • CD4 count = Number of immune cells in blood • Goal: normal range (500-1500 cells/mm3, median 900) • >200 cells/mm3 to prevent most opportunistic infections 41 CHOOSE 1 OF THE NEXT 3 SLIDES TO FOLLOW ALONG 42 43 Recommended Regimens for All Treatment Naïve Patients (9 regimens) NRTI Backbone Combination drug & class Darunavir/r Emtricitabine + Tenofovir (TDF) Raltegravir Elvitegravir/cobi Dolutegravir Elvitegravir/cobi Emtricitabine + Tenofovir alafenamide (TAF) INSTI Dolutegravir Raltegravir Darunavir Abacavir + Lamivudine PI Dolutegravir PI INSTI Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. 44 Recommended Regimens for All Treatment Naïve Patients (9 regimens) • INSTIs • 3 single tablet regimens • Stribild, Genvoya, & Triumeq • Raltegravir + Truvada or Descovy • Dolutegravir + Truvada or Descovy • PI • Darunavir + Truvada or Descovy Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. 45 Choosing a Regimen • Least adverse effects • INSTI-based • Durability • PI-based • Drug interactions • INSTI based (usually) • Single tablet regimen • INSTI- or NNRTI-based 46 47 48 55 Resources • AIDSinfo • http://www.aidsinfo.nih.gov • Guidelines and other resources • Centers for Disease Control and Prevention (CDC) • http://www.cdc.gov/hiv/ • Fact sheets, slide sets, testing and surveillance • World Health Organization • http://www.who.int/topics/hiv_aids/en/ • International data, facts and statistics • Positively Aware • http://positivelyaware.com/ • Annual HIV Drug Guide and other resources 56 Summary & Questions? • Many new therapies are available which give new options to patients seeking alternatives • All patients should be treated regardless of CD4+ count or viral load • Regimens should be individualized based on specific patient parameters • Adherence • Drug interactions • Adverse effects • Durability