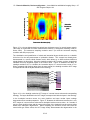
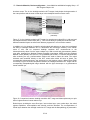
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PDF
Magnetic circular dichroism wikipedia , lookup
Diffraction topography wikipedia , lookup
Image intensifier wikipedia , lookup
Optical aberration wikipedia , lookup
Nonlinear optics wikipedia , lookup
Retroreflector wikipedia , lookup
Photonic laser thruster wikipedia , lookup
Silicon photonics wikipedia , lookup
Fiber-optic communication wikipedia , lookup
Ellipsometry wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Nonimaging optics wikipedia , lookup
3D optical data storage wikipedia , lookup
Surface plasmon resonance microscopy wikipedia , lookup
Night vision device wikipedia , lookup
Optical tweezers wikipedia , lookup
Phase-contrast X-ray imaging wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Interferometry wikipedia , lookup
Imagery analysis wikipedia , lookup
Hyperspectral imaging wikipedia , lookup
Super-resolution microscopy wikipedia , lookup
Confocal microscopy wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Chemical imaging wikipedia , lookup
27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Laser Medicine and Medical Imaging Group Academic and Research Staff Prof. James G. Fujimoto Visiting Scientists, Research Affiliates and Collaborators Prof. Mark Brezinski, Prof. Joel Schuman, Dr. Wolfgang Drexler, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Ingmar Hartl, Dr. Hiroshi Ishikawa, Dr. Christopher Kroeger, Dr. Xingde Li, Dr. Adelina Paunescu, Dr. Ping Xue, Nirlep Patel, Karl Schneider Graduate Students Aaron Aguirre, Ravi Ghanta, Pei-Lin Hsiung, Paul Herz, Tony Ko, Costas Pitris Technical and Support Staff Cindy Kopf, Mary Aldridge, Donna Gale Technology for Biomedical Imaging and Optical Coherence Tomography Sponsors Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-5-R01-CA75289-04 National Institute of Health Grant NIH-1-R01-AR44812-02 National Institute of Health Grant NIH-1-R01-HL63953-01 National Institute of Health Grant NIH-1-R01-EY131780-01 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Andrew M. Kowalevicz, Pei-Lin Hsiung, Paul Herz, Costas Pitris, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Hiroshi Ishikawa, Dr. Adelina Paunescu, Dr. Thomas Schibli, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Professor Franz X. Kaertner, Professor Mark Brezinski, Professor Joel Schuman, and Professor James G. Fujimoto Ultrahigh Resolution Optical Coherence Tomography Optical coherence tomography (OCT) is an emerging technology for micron-scale cross-sectional imaging of biological tissue [1, 2]. OCT is analogous to ultrasound imaging except that it uses light instead of sound. Imaging is performed by measuring the echo time delay of light. Since the speed of light is extremely rapid, it cannot be measured by direct electronic detection, but instead relies on low coherence interferometer. The longitudinal resolution in OCT images is inversely proportional to the optical bandwidth and proportional to the square of the center wavelength of the light source. The axial resolution in low 2 coherence interferometry is related to the bandwidth of the light source according to λ /∆λ. 27-1 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Ultrahigh resolution OCT requires extremely broad bandwidths because of this λ /∆λ dependence of the longitudinal resolution. This is particularly the case for the spectral region between 1.2 µm and 1.5 µm. This spectral region is of high interest for OCT because of the high penetration depth in biological tissue and the possibility to perform spectral resolved imaging of water absorption bands. Superluminescent diodes are used in conventional OCT systems and typically yield 10-15 µm longitudinal resolutions. We demonstrated previously OCT imaging with resolutions of 1 µm at 800 nm [3] and 5.1 µm at 1300 nm [4] in biological tissue using a Kerr-lens modelocked Ti:Sapphire laser with double-chirped mirrors and the self phase modulation broadened spectrum of a Kerr-lens modelocked Cr:Forsterite laser, respectively. 2 These broadband Kerr-lens modelocked lasers are not available commercially and require expensive and hard to fabricate double chirped mirrors. We tested a novel broad band light source that is continuum generation in air-silica microstructured fiber for the application in OCT. High nonlinearity, air-silica microstructure fibers [5] or tapered fibers [6] can generate an extremely broadband continuum using low energy femtosecond pulses. These fibers achieve high nonlinearities by using a tight mode confinement and shifting the zero of dispersion to shorter wavelengths. We successfully demonstrated the use of continuum generation in an air-silica microstructure fiber for OCT imaging [7]. We achieved a free-space axial resolution of 2.5 µm at a 1.3 µm center wavelength and demonstrated the application of the OCT system for in vivo imaging of biological tissue. These are the highest resolutions achieved to date for imaging in this wavelength regime. Figure 1 (left) shows a schematic of the experimental setup. We launched 100 fs pulses generated by a commercial Kerr-lens modelocked Ti:Sapphire laser , which was pumped by a frequency doubled Nd:Vanadate laser into a 1 m length of microstructure fiber. The fiber continuum was collimated with a microscope objective (MO), spectrally filtered by a long-pass filter and focussed with a chromatically corrected custom-designed lens (AL) into a dispersion shifted single mode fiber. A custom-designed lens and broadband 3dB fiber couplers (FC), designed for 1300 nm center wavelength (FC), were used in the OCT setup. Since the light source had excess amplitude noise, a dual balanced detection with two InGAs photodiodes (D1, D2) was used. Polarization controllers (PC) minimized the polarization mismatch of the interferometer arms to avoid degradation of the shape and the peak height of the interference fringes. Figure 1. (left) Ultrahigh resolution OCT system using continuum generation in an air-silica microstructure fiber as the light source. See text for explanation of the acronyms. (right) In vivo ultrahigh resolution (~6 x 2.5 µm, transverse x longitudinal resolution, 1.0 x 1.0 mm; 1000 x 3000 pixels) OCT image of a Syrian hamster cheek pouch. Different layers are clearly visible. We demonstrated the feasibility of in vivo ultra high resolution imaging by using the cheek pouch of a Syrian hamster, a well-established animal model for studies of cancer progression. Figure 1 27-2 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 (right) shows an in vivo image of cheek pouch which shows the epithelium, connective tissue and muscle layers at ultrahigh resolution. The cheek pouch was index matched by using a microscope cover glass and saline solution. While this approach achieves high resolutions in the important 1.3 µm wavelength range, there are several disadvantages. The femtosecond Ti:Sapphire laser used to generate the continuum requires a high power pump laser which makes the light source expensive and bulky. In addition, the microstructure fiber requires a free space coupling to the interferometer, which limits the optical power at the sample and the stability of the system. Finally, in order to achieve broad bandwidth, high-resolution operation, the OCT system used a delay line consisting of a galvonometer scanned retroreflector. This limits the maximum axial scan speed and real time imaging cannot be performed. To this end, we have demonstrated a novel light source for OCT using a Nd:Glass femtosecond laser with spectral broadening in a tapered single mode fiber. The Nd:Glass femtosecond laser is much less expensive (~5 times less) than the Ti:Sapphire femtosecond laser and pump. In addition, the Nd:Glass laser is compact (630mm x 320 mm x 160 mm) and can be more easily integrated into a robust and portable system for OCT imaging in the clinical setting. We have also designed a reflective grating phase delay line design that enables high speed, broad bandwidth optical delay scanning with this system. High speed and high resolution OCT imaging is achieved with 5 µm axial resolution and 90 dB sensitivity. Axial scan speeds of ~1 m/s are demonstrated, sufficient for real time imaging. OCT images taken with this light source and scanner were compared to images taken with a conventional semiconductor light source. Figure 2 demonstrates this comparison on an anesthetized African frog (Xenopus laevis) tadpole. The images consist of 676 axial pixels by 3000 transverse pixels, covering an area of 1 mm by 2 mm. The continuum light source yields an axial resolution of 5 µm in air or ~4 µm in tissue, which is four times higher than the conventional semiconductor source. This improved resolution enables the differentiation of small features which are indistinguishable with conventional light sources. Since the demonstration of OCT imaging with resolutions 5.1 µm at 1300 nm [4] in biological tissue using the self phase modulation broadened spectrum of a Kerr-lens modelocked Cr:Forsterite laser, we have worked toward the development of a compact and robust Cr:Forsterite laser for future implementation in a clinical OCT system. Recently, we have developed a compact (1’ x 2’ x 8”) Cr:Forsterite laser light source that can be integrated into a clinical OCT system. 27-3 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 0.25 mm x4 0.25 mm Figure 2. In vivo OCT image of an African frog tadpole (Xenopus laevis). (top) Conventional light source (AFC technologies) with 20 µm axial resolution in air (16 µm in tissue). (bottom) Continuum generated in tapered fiber as light source with 5 µm axial resolution in air (4 µm in tissue). We have also demonstrated ultrahigh resolution OCT imaging using fluorescence from a highly doped Ti:Sapphire crystal. Using a 5 W cw laser pumping the crystal, 40.3 µW of fluorescence with 138 nm bandwidth was coupled into a single-mode fiber (See Section I). This source enables ultrahigh resolution OCT imaging with 2.2 µm axial resolution in air (1.7 µm in tissue) and >86 dB sensitivity. Figure 3 demonstrates an OCT image taken with this source on an African frog (Xenopus laevis) tadpole in vivo. The image is constructed using C-mode scanning of 5 OCT images taken at different focal planes. The image consists of 600 transverse and 625 longitudinal pixels, covering a field of view of 600 µm and 470 µm. The ultrahigh resolution OCT image shows that individual cells and nuclei can be easily resolved. This demonstrates a simple and robust light source for spectroscopy and ultrahigh resolution OCT imaging without the need for complex femtosecond Ti:Sapphire lasers. Figure 3. Ultrahigh resolution OCT image of an African frog tadpole (Xenopus laevis) taken with Ti:Sapphire fluorescence. Imaging was performed with <2 µm axial resolution and 5 µm transverse resolution. Five OCT images with different focal depth settings was fused to form this image of extended depth of field 27-4 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Spectroscopic Optical Coherence Tomography Spectroscopically resolved imaging is important because it promises to enable imaging of functional properties of tissues on a micron scale by using spectroscopic properties. Relatively few studies on spectroscopic OCT have been performed because broadband light sources have not been available. An earlier study of spectroscopic OCT used a bandwidth of ~50 nm at 1.3 µm [8]. We recently used a Kerr-lens modelocked Ti:Sapphire laser with dispersion compensation by double chirped mirrors for spectroscopic imaging in the wavelength range from 650 to 1000 nm [9]. Previous spectroscopic OCT studies in the spectral region of the water absorption band at 1.45 µm used two different light sources in dual wavelength OCT systems. One light source was used for absorption measurements and the second light source for referencing [10, 11]. However, this approach adds the noise of two independent light sources, and accurate referencing is hard to achieve. In order to demonstrate spectroscopic OCT imaging, we investigated differential water absorption by selecting a spectral range of 200 nm centered at 1400 nm from the continuum. We imaged a phantom consisting of two microscope cover glasses of 170 µm thickness confining a water sample. A schematic of the setup is shown in Figure 4 (left). The cover glasses were in contact at one side of the sample and had a spacing of ~0.8 mm at a lateral distance of 10 mm. The focal position of the imaging beam was ~0.4 mm above the bottom coverglass. Figure 4. (left) The phantom consists of two microscope cover glasses enclosing H2O as absorber. (center) Digitally demodulated OCT image of the phantom used for this study. (right) Spectrum of the OCT echo of the cover glass surface at the points indicated with A and B and one intermediate point. The arrow indicates wavelength of the water absorption band. The full interference signal was digitally recorded at 12 bit resolution with 30,000 digitalization points per 1.3 mm A-scan. A digitally demodulated image of the sample is shown in Figure 4 (center). The spectrum of several A-scans was calculated by Fourier-transforming a 2000 point window centered at the maximum of the reflection signal. The spectra obtained were normalized to the intensity maximum. The spectra at the points A and B and one intermediate point are shown in Figure 4 (right). One clearly observes that the absorption of the spectral intensity around 1.45 µm increases with increasing absorber thickness. Novel Imaging Devices for Optical Coherence Tomography The success of optical coherence tomography in clinical applications will depend in large part on the design and availability of delivery mechanisms that allow seamless integration of OCT with existing diagnostic imaging modalities. OCT has the advantage that it is fiber optically based and can readily be integrated with a wide range of existing clinical imaging instruments such as microscopes, laparoscopes, endoscopes, and catheters. As an imaging technique capable of imaging human tissue at high resolution, OCT could lead to detection of pathology at earlier stages than currently possible, leading to improved patient prognosis. Several imaging devices 27-5 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 have been designed and evaluated to investigate the feasibility of OCT for the clinical assessment of pathology of a variety of non-transparent tissue in vivo. Linear and rotational scanning catheters Since optical coherence tomography is fiber optically based, it can be easily integrated into a wide variety of clinical imaging devices. We have developed a series of OCT catheters that are being used in several ongoing clinical studies. We have developed a small diameter OCT catheter, which can be used either for linear scan or radial scan. The OCT catheter consists of an optical coupling element at its proximal end, a single-mode optical fiber running its length, and optical focusing and beam directing elements at the distal end. The single-mode optical fiber is in a flexible hollow metal cable that is either rotated or translated with a motor drive unit at the proximal end. A schematic and a photograph of the distal end of the OCT catheters is shown in Figure 1. The distal end contains a gradient index lens and a right-angle microprism to focus and direct the beam at a 90° angle with respect to the axis of the catheter. For our studies we chose the optics for the catheter to achieve a spot size (transverse resolution) of ~13 µm and a ~2-3 mm working distance. The fiber optic cable and distal optics are protected by a sealed transparent plastic sleeve which remains stationary as the fiber optic cable and optics move inside. The catheter has an outside diameter of ~1 mm and can be passed through the working channel of a standard endoscope. The linear scan probe scans the longitudinal position of the OCT beam at a fixed angle, to generate a rectangular image of a longitudinal plane at a given angle with respect to the probe. The radial scan probe directs the OCT beam radially outward and scans the angle of emission rotationally to generate an image which is displayed in a polar plot. transparent guard microprism SM fiber sheath 500 µm Guard ...... hollow metal cable Photo of Distal End (on US Penny) Aiming Beam GRIN lens silicone sealant Figure 5. Schematic and photograph of the OCT catheter distal end. The metal speedometer cable, focusing gradient index lens, and right-angle prism are protected by a metal guard and encased within a transparent sheath. Both linear and radial-scanning OCT catheters have their advantages and limitations. In OCT imaging catheters, the focused beam spot size (transverse resolution) and working distance are determined by the distal micro-optics. The beam is in focus when the tissue is positioned near the focal position of the beam within the depth of field. Images acquired with the linear-scanning catheter are displayed in Cartesian (rectangular) coordinates, whereas images acquired with the radial-scanning catheter are displayed in polar (circular) coordinates. The linear scanning approach has the advantage that the pixel spacing in the transverse direction is always uniform. This contrasts with the radial imaging approach where the transverse (circumferential) pixel spacing increases with increasing distance from the catheter. Thus, the radial OCT images become progressively coarser when large diameter lumens are scanned. To image with the linear scan catheter design, the distal end is positioned against the wall of the lumen. Placing the catheter in contact with tissue not only fixed the distance to the tissue, but also helped stabilize the catheter position during cardiac or respiratory movements. The radial scan method is analogous to that used in intravascular ultrasound catheters (IVUS) which contain a rotating distal ultrasonic transducer. The radial scanning catheter is well suited 27-6 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 for imaging small (few mm) diameter lumens such as human arteries or the gastrointestinal tract of animals. However for large diameter lumens such as the human esophagus, it is difficult to position and stabilize the radial-imaging catheter in the center of the lumen. Movements of the lumen or endoscope resulted in motion artifacts and variations in the focus position. If radial imaging were performed with the catheter de-centered in the esophagus and near the mucosal surface, only a small sector of the 360° scan range would be in focus. Doppler imaging catheter It is possible to combine functional as well as structural imaging. For intravascular imaging, the measurement of blood flow is especially important for assessing athersclerotic narrorwing of arteries and the effects of atheractomy therapy. We have developed a Doppler OCT catheter integrates functional imaging with structural imaging using OCT. The catheter consists of an angle-cleaved single mode optical fiber with a gradient index (GRIN) lens glued to the angle cleaved end of the fiber to focus the beam. In order to detect a Doppler signal due to flow, a nono zero projection of the imaging beam along the longitudinal axis of the catheter is needed. A ~60 o microprism is custom-made and employed so that the beam exits the catheter at ~60 with respect to the catheter axis. The single mode fiber, the GRIN lens and the microprism are attached to form a single unit and housed concentrically in a 27 Gauge (~410 µm) hypodermic metal guard. The entire catheter is housed within a transparent plastic sheath with a sealed distal end. At the proximal end of the catheter the hollow wire is connected to a DC motor. The optical beam can be scanned radially by rotating the hollow metal wire along with the single mode fiber and the distal optics. A rotary coupler based upon a glass capillary tube is used to connect the rotating fiber at the proximal end of the catheter and a stationary fiber from the light source [12]. Figure 6 shows one representative schematic of the catheter and a schematic of the catheter demonstrated for measuring the intraluminal flow profile in phantom. Catheter Sheath beam 60o 3.17 mm 0.41 mm 1.14 mm A B flow tube Figure 6. (a) Schematic of the Doppler OCT imaging catheter. The beam exits from the catheter o o at an angle of 48 in air (60 in water) with respect to the catheter’s longitudinal axis. (b) Schematic of the experimental phantom consisting of a clear plastic tube with an inner diameter of 3.17 mm. 1% Intralipid is circulating through the tube at a constant flow rate. The Doppler catheter can be used for assessment of intraluminal blood flow within a vessel as well as structural imaging of the fine structures within the vessel wall. The Doppler OCT catheter can also be potentially used for evaluating the severity of stenosis and the outcome of cardiovascular interventions such as stenting. We are currently evaluating the potential of this device for deducing the vessel wall diameter in a physiological relevant environment (blood). This device can also be used for imaging the fine structures within the vessel wall when moderate saline flushing is used. In addition to the flow velocity profile, the flow turbulence may also be quantifiable by investigating the flow velocity variance. Future work will also focus on investigating the potential of this device for diagnosis of thrombosis or aneurysm from the velocity variance assessment. 27-7 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 OCT Biopsy Needle OCT applications in the past have been limited to the surfaces or lumens of organ systems because of the ~2-3 mm penetration depth of OCT in most tissues. Pathologies within solid tissues or organs have been outside the range of OCT. There are, however, many clinical scenarios where high resolution imaging of solid tissues is desirable. One promising application of OCT is in imaging pathology and guiding biopsy in solid tissues. This procedure could reduce the sampling error of excisional biopsy in diagnosing cancers in solid organs such as the prostate or breast. Other applications include optical imaging in scenarios where excisional biopsy is hazardous, and in surgical guidance such as in cryosurgery or interstitial photodynamic therapy. We have developed a prototype of an interstitial imaging needle for OCT that has a diameter as small as 27 Gauge (~410 µm) [13]. A GRIN lens and a micoprism are used to focus and deflect the optical beam which is emitted at an angle of 90 degrees from the axis of the needle. The optical fiber is housed concentrically within a thin wall stainless steel hypodermic tube with a 27 gauge outer diameter. This hypodermic tube protects the optical fiber and facilitates the insertion of the needle into the tissue. The imaging needle has a confocal parameter of ~380 µm, corresponding to a spot size (or transverse resolution) of ~17 µm, with a focal distance ~80 µm outside the optical window. The optical beam can be scanned radially by rotating the entire needle along with the distal optics. This technique is similar to that used with acupuncture needles. The OCT imaging plane is perpendicular to the needle axis and the position of the imaging plane can be controlled by varying the depth of needle insertion. The OCT imaging needles can be inserted into tissue independently or coupled to standard biopsy techniques, such as fine needle aspiration (FNA) or core biopsy. Figure 7 shows one concept of OCT guided biopsy. The OCT needle is introduced first, prior to the biopsy device. If an area of tissue pathology is detected, the biopsy device would then be inserted in parallel, riding the OCT needle in a monorail-like fashion. This approach has the advantage of allowing selective insertion of the larger 18 gauge biopsy devices only when deemed necessary by the imaging. Since the small 27 gauge OCT imaging needle would produce only minimal trauma, more needle insertions could be performed than currently possible, allowing larger volumes of tissue to be screened. Biopsy Imaging biopsy specimen Imaging Volume Figure 7. Monorail biopsy and OCT imaging needle. A monorail design takes advantage of the small size (27 gauge) of the OCT imaging needle, allowing imaging to be performed prior to the introduction of an 18 gauge biopsy needle. This approach reduces trauma, allowing multiple sites to be imaged before a decision to biopsy is made. Needles of even smaller diameter (200 µm or less) can ultimately be built. The movement of the needle can be performed with minimal resistance from the tissue and minimal trauma. OCT imaging can assess larger volumes of tissue than are possible to excise and imaging can be performed in real time prior to tissue biopsy with minimal tissue trauma. The OCT imaging needle and OCT needle biopsy device would permit the imaging of pathology inside virtually any solid organ or tumor and permit the guidance of biopsy based on microstructural features. This would be an enabling technology for a wide range of research and clinical applications. 27-8 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Surgical Imaging Probe The development of intraoperative OCT imaging enables pathology to be visualized in situ and in real time in tissues that were previously inaccessible in vivo. Techniques that can visualize microscopic disease in situ and in real time promise to improve diagnostic accuracy, and the ability to visualize tissue structures intraoperatively should also facilitate the guidance of microsurgical procedures. Imaging in the surgical suite requires a delivery device that is compact, robust, and sterilizable using standard hospital sterilization techniques. We have designed and developed a handheld probe and demonstrated its application for imaging the prostate during nerve-sparing radical prostatectomy and for imaging cartilage during open field surgery. The compact hand held probe consists of a Hopkins lens relay and a galvanometer mirror which steers the beam in the transverse direction with the scanning range and spot size magnified by telescope optics. The current imaging probe has a 2 cm working distance which allows the surgeon to maneuver the beam within the tight confines of the surgical field. A low power green aiming beam allows the surgeon to localize the imaging area and facilitate marking for correlation with histology. The probe has a stainless steel outer tube with a transparent window which can be sterilized using standard hospital sterilization procedures and slipped over the distal end of the probe prior to imaging. The proximal end of the probe including cables can be enclosed in a sterile surgical camera bag. The current probe scans over 3.5 mm and produces a focused spot with an 18 µm transverse resolution. The transverse resolution and scan range are determined by the choice of lenses in the relay system. If necessary, a small coherent fiber bundle fiberscope can be used in conjunction with the hand held probe to allow direct visualization of the region being OCT imaged. A version of the probe has recently been designed that uses two orthogonally positioned galvanometer mirrors to enable scanning of the OCT beam in the en face plane. Current studies are underway using this probe for in vivo investigations of optical coherence microscopy, a technique that combines standard OCT imaging with confocal microscopy. An additional ongoing study includes using the probe with a portable OCT system at the National Cancer Institute to image oral leukoplakia and monitor pharmacological chemoprevention therapy. Optical Coherence Microscopy Sponsors Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-5-R01-CA75289-04 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Pei-Lin Hsuing, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Ingmar Hartl, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Professor Mark Brezinski, and Professor James G. Fujimoto Optical Coherence Tomography (OCT) has exciting potential for high resolution, in vitro and in vivo optical biopsy in several types of scattering tissue [2]. OCT combines the coherence gating afforded by broadband light sources with heterodyne detection to provide superior signal to noise and image contrast at a range of wavelengths. Standard clinical OCT provides cross-sectional imaging with 10-15 µm axial and transverse resolution. High-resolution OCT has 1-2 µm axial 27-9 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 resolution and 5-6 µm transverse resolution, but is limited in the axial scan range due to the small confocal parameter of the sample beam. Like OCT, laser scanning confocal microscopy (LSCM) has also been demonstrated as a potential technique for high-resolution imaging of tissue microstructure [14]. LSCM uses high-magnification, high numerical aperture objective lenses to provide 1-3 µm transverse resolution and better than 1 µm axial sectioning capability. In contrast to OCT, confocal microscopy samples an en face scan plane. Figure 8 compares cross-sectional and en face imaging planes. Transverse X Transverse X DEPTH PRIORITY EN FACE Incident Beam Incident Beam z Axial z Axial (Range Depth) y (Range Depth) y Figure 8. Schematic comparing scanning modalities for Optical Coherence Tomography (OCT) and Laser Scanning Confocal Microscopy (LSCM). OCT uses depth scanning to form crosssectional imags. LSCM uses transverse scanning to form en face images. LSCM has shown particular promise for in vivo imaging of cellular structure in a human tissues [14], but signal attenuation in scattering media limits imaging depths to less than 1 mm. To extend and improve upon confocal microscopy, our group demonstrated in 1994 the concept of a new technique called optical coherence microscopy (OCM) [15]. OCM combines the coherence gate with the confocal gate to improve rejection of unwanted scattered light that degrades image contrast. With the superior signal to noise ratio offered by coherence-gated techniques, OCM can yield approximately 2x greater imaging depths than standard confocal microscopy. Realtime, in vivo OCM has since been demonstrated for imaging cellular structure in human skin [16]. As with axial resolution in OCT, rejection of unwanted scattered light in OCM improves with shorter coherence length, higher bandwidth light sources. Our recent work on high-resolution OCM has focused on extending the technique to perform high speed imaging with broadband light sources at multiple wavelengths. One major current limitation to achieving high resolution OCM over a range of wavelengths is the lack of high speed, broadband phase modulators. Previous studies have used either stretched fiber phase modulators, which have limited scanning speeds, or waveguide phase modulators, which have limited bandwidths. Our group developed and demonstrated grating phase delay scanners for high speed OCT imaging [17]. These systems are similar to grating-based pulse amplitude and phase shaping techniques in femtosecond optics [18]. The devices use a grating and lens to produce spectral dispersion combined with a galvonometer-controlled mirror to produce a phase shift in the Fourier plane, resulting in a group or phase delay. The grating phase delay scanner enables group and phase delays to be independently controlled. Recently, grating phase delay scanners have been demonstrated to achieve phase modulation [19]. 27-10 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 T o p V iew C urved m irro r G ratin g G ratin g P h ase M o d u lato r In R eflective G eo m etry D ou b le p ass m irro r B ro ad b an d S o u rce +- S can n in g M irro r D etection E lectro nics D1 In p ut b eam reflected fro m m irro r D o u b le p ass m irror 50/50 D2 M o n ito r S id e V iew C u rved m irro r P o larizatio n C on trol C o m p uter M irror S can n in g M irro r S -V H S R eco rd er f2 f f3 G ratin g f1 f2 + f3 4 F u n ctio n G enerato r G alvo C on trollers x y m o d u lato r sam ple O b jective 30x 0.9 N A f3 H an d -H eld X -Y S can n in g P ro b e f2 y-g alvo x-g alvo Figure 9. Optical coherence microscopy system using a reflective phase delay scanner and a compact hand-held probe. A broadband light source is split into reference and sample beams by a 50/50 fiber optic coupler. The sample beam passes through a reflective grating phase modulator while the sample beam is focused onto the specimen using a novel hand-held probe. The returned beams are recombined at a dual-balanced electronic receiver integrated with a PC and S-VHS recorder. The reflective grating phase modulator supports broad bandwidth, high axial resolution imaging and also enables real time imaging at a wide range of wavelengths. We designed and demonstrated OCM using a novel reflective grating phase modulator and a compact handheld probe. Figure 9 shows the experimental setup for the optical coherence microscopy system. Our grating phase modulator is similar to reflective pulse stretchers and compressors previously developed for femtosecond pulse amplification [20]. The reflective phase delay line can support broad spectral bandwidths and perform high-speed phase modulation. This system design will enable high resolution, high speed OCM imaging at wavelengths which were previously inaccessible. We acquired preliminary data using a superluminescent diode laser source with center wavelength of 1300nm and 65 nm bandwidth. We achieved transverse resolution of better than 3.3 µm and demonstrated in vivo imaging on an African frog tadpole (Xenopus laevis) (Figure 10). The tadpole was imaged from the dorsal side with 1375x500 and 1375x250 pixel resolutions at 2 to 4 frames per second, respectively. Cellular structure was clearly visible at multiple en face imaging depths. The cell nuclei and cell borders appear highly scattering. In addition, circulatory flow in a large vessel was also visible in sequential images or video. To demonstrate materials imaging, OCM imaging was performed on laser fabricated optical waveguides in glass. The waveguides are inside the glass and normally require phase contrast microscopy for visualization. 27-11 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Figure 10. OCM en face images taken of Xenopus laevis tadpole and of a laser-fabricated waveguide embedded in glass (c). Images a, d-e are 1375x250 pixels taken at 4 frames per second, while images b and c are 1375x500 pixels taken at 2 frames per second. The field of view in the images is 130 µm x 140 µm. We will combine our work on broad-bandwidth light sources with our new system design to achieve high-resolution OCM at multiple wavelengths. We believe the system will provide a powerful tool for optical biopsy to explore the limits of coherence-gated imaging for clinical diagnostic purposes. Ultrahigh Resolution Ophthalmic Imaging Sponsors National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-1-R01-EY131780-01 National Institute of Health Grant NIH-5-R01-CA75289-04 Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 Air Force Office of Scientific Research Grant F49620-98-01-0084 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Andrew M. Kowalevicz, Pei-Lin Hsuing, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Hiroshi Ishikawa, Dr. Adelina Paunescu, Dr. Thomas Schibli, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Dr. Uwe Morgner, Professor Franz X. Kartner, Professor Mark Brezinski, Professor Joel Schuman (New England Eye Center), and Professor James G. Fujimoto OCT has perhaps been most widely investigated in ophthalmology, where it is beginning to make a clinical impact in the assessment of retinal diseases such as macular holes, age-related macular degeneration, glaucoma, and diabetic retinopathy [1, 21-23]. Current clinical practice 27-12 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 calls for the development of techniques to diagnose ophthalmic disease in its early stages, when treatment is most effective and significant irreversible damage can either be prevented or delayed. With typical axial resolutions of 10 µm, OCT already provides more detailed structural information than any other conventional imaging technique. However, the detection of many of the early changes associated with diseases can require more accurate quantification of retinal structure than is possible with standard resolution OCT. 250 µm Figure 11. In vivo standard resolution (top) and ultrahigh resolution (bottom) OCT images of a normal human fovea at approximately the same site. Resolutions were 10-15µm (axial) x 15µm (transverse) and 3µm (axial) x 15µm (transverse) respectively. Using the broad bandwidth of our ultrahigh resolution OCT system, we can image with axial resolutions greater than 3 µm (in the retina), corresponding to a factor of 5 improvement over OCT technology using superluminescent diode sources. The signal to noise ratio for the system is ~100 dB. This system enables a significant improvement in the visualization of intraretinal structures for earlier diagnosis and more precise staging of pathology (Figure 11). To our knowledge, the image shown in Figure 11 represents the highest resolution in vivo image ever acquired of the human retina. Ultrahigh resolution OCT offers an unprecedented visualization of retinal morphology with structures such as the retinal nerve fiber layer, retinal pigment epithelium, and the inner and outer plexiform layers. These structures are relevant in a variety of retinal diseases, including age-related macular degeneration, diabetic retinopathy, and glaucoma (the three leading causes of blindness worldwide). Nasal Temporal Thickness (µm) 250 µm 350 250 Henle’s and Photoreceptor Layer Thickness Retinal Thickness 150 50 -3.0 Ganglion/NFL Layer Thickness 0 3.0 Transverse Position (mm) Figure 12. Ultrahigh resolution allows for an unprecedented visualization of intraretinal structures that may be quantified to provide an objective measure of retinal disease. 27-13 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Image processing techniques can be applied to acquired tomograms to quantify retinal and intraretinal structures relevant to disease. Figure 12 illustrates the application of preliminary image processing segmentation algorithms to ultrahigh resolution OCT images to quantify retinal and intraretinal structures. Ultrahigh resolution enables the quantification of layers relevant to retinal disease, which were previously not visualized or quantified using standard resolution OCT. Precise quantification of the retinal thickness is important for the diagnosis and staging of macular edema and diabetic retinopathy. Quantification of the photoreceptor and Henle’s layer may be important in a variety of retinal diseases. Quantification of the ganglion cell layer and the nerve fiber layer is important in retinal diseases such as glaucoma. Figures 13-15 demonstrate the ability use the ultrahigh resolution OCT to quantify and map the different retinal layers in a normal volunteer. 1.5 Superior 0.5 Retinal Thickness (µm) Nasal 0 Temporal Horizontal Position (mm) 1.0 -0.5 -1.0 -1.5 -1.5 Inferior -1.0 -0.5 0 0.5 1.0 1.5 Transverse Position (mm) Figure 13. In vivo two-dimensional retinal thickness map of a normal human macula. This image is constructed from 4200 measurement locations (7 tomograms consisting of 600 A-Scans each). The transverse sampling resolution was 5 µm and the horizontal sampling resolution was 500 µm per pixel. Superior 1.0 0.5 0 Nasal Center Horizontal Position (mm) 1.5 -0.5 Nerve Fiber Layer Thickness (µm) -1.0 Inferior -1.5 0 1.0 2.0 3.0 4.0 5.0 Transverse Position (mm) Figure 14. In vivo two-dimensional nerve fiber layer thickness map along the papillomacular axis of a normal human volunteer. This image is constructed from 6000 measurement locations (10 tomograms consisting of 600 A-Scans each). The transverse sampling resolution was 5 µm and the horizontal sampling resolution was 520 µm per pixel. 27-14 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 1.5 Superior 0.5 Nasal 0 Temporal Horizontal Position (mm) 1.0 GCL+IPL Thickness (µm) -0.5 -1.0 -1.5 -1.5 Inferior -1.0 -0.5 0 0.5 1.0 1.5 Transverse Position (mm) Figure 15. In vivo two-dimensional intra-retinal layer thickness map of a normal human macula. This image in constructed from 4200 measurement locations (7 tomograms consisting of 600 AScans each). The transverse sampling resolution was 5 µm and the horizontal sampling resolution was 500 µm per pixel. The visualization and quantification of retinal and intraretinal layers should serve as a valuable clinical tool for the early assessment of ophthalmic disease. This concept has already been demonstrated in a mouse retinal disease model, which allows us to follow and track different retinal diseases in this animal. Using the ultrahigh resolution OCT system, we have imaged and identified the many intraretinal layers of the mouse retina. Figure 16 illustrates the ability for ultrahigh resolution OCT to visualize the intraretinal layers of a normal mouse retina in vivo. When compared with histology taken from the same animal, the ultrahigh resolution OCT image corresponds well with the layers identified in the histology. NFL IPL INL OPL ONL RPE 100 µm Figure 16. In vivo ultrahigh resolution OCT image of a normal mouse retina and corresponding histology. The layers identified in the OCT images correspond well with the layers in the histology In the rhodopsin knockout mouse, the outer plexiform and outer nuclear layers undergo degeneration three months postpartum. Figure 17 illustrates the differences between the in vivo OCT images of a normal mouse retina and a rhodopsin knockout mouse retina. At 5 months of age, the outer plexiform layer and the outer nuclear layer of the rhodopsin knockout mouse retina would have undergone degeneration. When comparing the knockout mouse retina with the normal wild type mouse retina, the OCT image clearly demonstrates this degeneration in the 27-15 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 knockout mouse. The in vivo ultrahigh resolution OCT images clearly depict the degeneration of the outer plexiform and the outer nuclear layer in the rhodopsin knockout mouse retina. IPL INL OPL 50 µm ONL RPE Rd -/- Knockout (5 months) Rd +/+ W ildtype Figure 17. In vivo ultrahigh resolution OCT image of a normal mouse retina (Rd +/+ wild type) and a rhodopsin knockout mouse retina (Rd -/-). OCT has the ability to quantify the thickness of the different intraretinal layers as well as track disease progression in a non-invasive manner. In addition to in vivo imaging of retinal in animal models and patients, we have also investigated the ability of ultrahigh resolution OCT to discriminate the microscopic anatomy of the human retina in vitro, and we compared ultrahigh resolution OCT measurements to the histomorphometry taken from the same cadaver eye. Both normal and glaucomatous cadaver eyes were obtained from National Disease Research Interchange (NDRI) and New England Medical Center (NEMC) were fixed in a 3% glutaraldehyde solution. To minimize subretinal fluid after sectioning the eye, perfluorocarbon solution was used to flatten the retina. The retinal thickness was measured directly on the OCT scans taken within 48 hours of death. The corresponding histomorphometry was performed on this histology using a Nikon Elipse E400 microscope. Figure 18 shows comparison between the ultrahigh resolution OCT image and the corresponding histopathological image obtained with the light microscope on a glaucomatous human cadaver eye. Figure 18. Comparison between ultrahigh resolution OCT image and histomorphometry on optic disk of a glaucomatous human cadaver eye. Retinal layers, including the nerve fiber layer, inner nuclear layer, outer nuclear layer, the retinal pigment epithelium, choriocapillaris and choroids could be identified. The correspondence of retinal layers between the ultrahigh resolution OCT image and the histopathology is presented in 27-16 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Figure 19. Ultrahigh resolution OCT and histomorphometry produced similar retinal thickness data in normal and glaucomatous eyes. We found differences in retinal thickness between normal and glaucomatous eyes in both ultrahigh resolution OCT and histomorphometry. Figure 19. Correlation between retina layers obtained with the ultrahigh resolution OCT and light microscopy on glaucomatous human cadaver eye. The images represent the same section of the retina of the same eye. These studies demonstrate that ultrahigh resolution OCT can be used to identify different intraretinal layers, quantify layer thickness, and track retinal disease progression. Future work will involve ultrahigh resolution OCT imaging studies of patients with retinal diseases such as age-related macular degeneration, diabetic retinopathy, and glaucoma. Ultrahigh-Resolution and Spectroscopic OCT Imaging of Gastrointestinal Malignancies Sponsors National Institute of Health Grant NIH-5-R01-CA75289-04 Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 Air Force Office of Scientific Research Grant F49620-98-01-0084 National Institute of Health Grant NIH-2-R01 EY11289-15 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Andrew M. Kowalevicz, Pei-Lin Hsiung, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Dr. Hiroshi Mashimo and Dr. Muthoka Mutinga (VA Medical Center), Dr. Jacques Van Dam (Stanford U.), Professor Mark Brezinski, and Professor James G. Fujimoto 27-17 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Endoscopic OCT has already been performed by our group using linear and radial scanning methods to demonstrate the ability to discern changes in architectural morphology associated with Barrett’s esophagus [24]. This study has demonstrated that endoscopic OCT can differentiate normal from Barrett’s epithelium in real-time based on differences in epithelial architecture. This suggests that OCT may be used as an adjunct to endoscopy for screening and surveillance of Barrett’s esophagus, in particular in situations where endoscopic visualization is difficult, such as to detect short segment Barrett’s or as a follow up to photodynamic therapy to detect residual islands of Barrett’s pathology. However, the image resolution of conventional OCT (~10-15 µm) is sufficient to differentiate architectural but not cellular morphology. We have performed in vitro ultrahigh-resolution OCT and spectroscopic OCT on human esophagus as a first step towards an ultrahigh resolution and spectroscopic endoscopic OCT imaging clinical system. Ultrahigh resolution potentially allows visualization of tissue structure at the cellular level, which could facilitate Barrett’s detection. Spectroscopic OCT was investigated as a modality for improving contrast and differentiation OCT images. This technique may help differentiate regions of different spectroscopic optical backscattering properties that are associated with different tissue morphologies. Imaging of in vitro specimens was performed using a bench top ultrahigh-resolution OCT system [3]. This state-of-the-art OCT system performs imaging using a femtosecond Ti:Al2O3 laser light source that emits ~5 fs optical pulses, corresponding to bandwidths of up to 350 nm at a center wavelength of 800 nm. The OCT system was designed to support up to 260 nm of bandwidth, achieving 1.5 µm axial resolution in free-space or ~1.1 µm in tissue. This is an order of magnitude improvement in resolution compared to conventional OCT systems. An achromatic lens with a 10 mm focal length was used to focus the beam on the in vitro tissue specimens to achieve 5 µm transverse resolution. This system was used to image in vitro Barrett’s esophagus specimens to demonstrate the potential of ultrahigh-resolution OCT imaging. Spectroscopic OCT imaging is an extension of ultrahigh-resolution OCT which utilizes the broad spectral bandwidth of the optical source to obtain information from the spectral content of the backscattered light [9]. Conventional OCT imaging detects the envelope of the interference signal which is generated by the reflections of light from the reference and sample arms. The amplitude of this envelope is used to display the intensity of optical backscatter from specific regions within the tissue. Spectral information can be obtained by digitizing the full interference signal and using digital signal processing techniques. Three surgical specimens of human esophagus were used. Imaging was performed only on discarded tissue specimens. Specimens were maintained in a 0.9% saline solution and imaged with ultrahigh-resolution and spectroscopic OCT within 3 hours of resection. The tissue specimen was placed on a three-dimensional, computer-controlled, micron-precision translation stage. Imaging was performed using the OCT microscope beam delivery apparatus. Immediately following OCT imaging, the specimen was marked with India ink spots to indicate the OCT image plane location. Specimens were placed in 10% Formalin for at least 24 hours prior to standard histological processing. Histological slides were prepared from 5 µm thick sections stained with hematoxylin and eosin. OCT images were compared with corresponding histological images. Figure 20 (a) is an OCT image of Barrett’s epithelium. The image size was 2 mm x 1 mm (1000 x 1500 pixels). The image resolution was 1.1 µm axial and 5 µm transverse. Figure 20 (b) shows corresponding histology from the same site. Multiple crypts and glandular structures were observed along the surface. Magnified images of selected regions are shown in Figure 20 (c) and 20 (d). Isolated regions of low and high optical backscatter were observed. Regions of low backscatter corresponded to fluid-filled crypts or glands. It is unclear if individual cellular features are visible, although the localized points of high backscatter are suggestive of nuclei. 27-18 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 D C A 300 µm C 100 µm B 300 µm D 100 µm Figure 20. Ultrahigh-resolution OCT imaging of Barrett’s esophagus in vitro. (a) OCT image acquired at ~1.1 µm axial and 5 µm transverse resolution illustrating multiple crypt- and gland-like structures. (b) Corresponding histology confirms architectural morphology observed in the OCT image. (c,d) Magnified regions from sites indicated in (a). Localized regions of high optical backscatter (dark pixel clusters in image) may represent individual cell nuclei. Spectroscopic OCT was performed on regions of normal and Barrett’s esophagus in vitro to assess if spectroscopic imaging could enhance image contrast or improve the differentiation of pathology. Amplitude tomograms represent the intensity of backscatter light, while spectroscopic tomograms (Figure 25) measure the spectral content of the backscattered light. For the spectroscopic OCT images presented here, the spectral shift of the backscattered light was measured by calculating the center of gravity of the spectrum from each pixel in the image. The spectral shift is mapped to a false color scale and plotted as hue, while the intensity of the backscattered light is plotted as saturation, with luminance kept constant. The false color image thus provides an indication of the spectral shift of the backscattered light as well as its intensity. A 250 µm B 250 µm Figure 21. Spectroscopic OCT imaging. Green and red hue color scale indicates enhanced optical scattering of short and long wavelengths, respectively. (a) Spectroscopic OCT image of transition zone between normal squamous epithelium (right) and pathologic columnar epithelium (left). For normal epithelium, note the relatively smooth color transition from green to red with 27-19 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 increasing depth in tissue. (b) Spectroscopic OCT image of Barrett’s esophagus. The irregular epithelial morphology from crypt- and gland-like structures are indicated by areas of red hue (enhanced long-wavelength scattering) near the surface (arrows). Figure 21 compares spectroscopic OCT images of normal and Barrett’s esophagus. The epithelium has a green hue in the false color image, indicating enhance scattering of shorter wavelengths of light from this layer. Immediately below the epithelium, the lamina propria contains focal areas of red hue, indicating regions of enhanced longer wavelength scattering. Since longer wavelengths penetrate more deeply into tissue, it is expected that shorter wavelengths will be scattered more from structures near the surface while longer wavelengths are scattered more from deeper structures. Therefore, a shift of hue from green to yellow to red should occur with depth. Because normal squamous epithelium is relatively homogeneous, this gradual transition from green to yellow to red is observed in Figure 25 (a). This is in contrast to Figure 25 (b) where the presence of multiple crypt and glandular structures along the surface disrupt the morphological structure. The spectroscopic tomogram in Figure 25 (b) shows no significant degree of uniform green hue (short wavelength scattering) within the epithelium. Instead, more frequent focal areas of red hue (long wavelength scattering) are present. Occasional red focal areas are present at the surface as indicated by arrows. Increased disruption of the normal squamous architectural morphology from the formation of crypts, glands, and potentially cellular structures such as increased number and size of nuclei, disrupt the normal spectroscopic scattering behavior in the spectroscopic OCT image. In conclusion, ultrahigh-resolution OCT techniques achieved 1.1 µm image resolution in in vitro specimens and showed enhanced resolution of architectural features. Spectroscopic OCT identified localized regions of wavelength-dependent optical scattering, enhancing the differentiation of Barrett’s esophagus. These techniques improve structural tissue recognition and suggest the future potential for resolution and contrast enhancements in clinical studies. We are currently designing a new catheter compatible with the broadband spectrum of the light source, which will allow us to perform in vivo endoscopic ultrahigh-resolution OCT and spectroscopic OCT. These studies were performed in collaboration with Dr. Jacque van Dam at Stanford Medical School, Dr. Muthoka Mutinga and Dr. Hiroshi Mashimo at the Veterans Administration Medical Center. Ultrahigh Resolution Optical Coherence Morphology and Tissue Preservation Sponsors National Institute of Health Grant NIH-5-R01-CA75289-04 Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-1-R01-AR44812-02 National Institute of Health Grant NIH-1-R01-HL63953-01 27-20 Tomography Studies of Tissue 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Pei-Lin Hsuing, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Xingde Li, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Professor Franz X. Kartner, Professor Mark Brezinski, Dr. Michael Weinsterin (Brigham and Women’s Hospital), and Professor James G. Fujimoto To date, many previous studies have compared ex vivo OCT imaging to histopathology. While some tissues, such as arterial pathology or cartilage, are relatively stable post mortem, others, such as epithelial tissues, exhibit rapid degradation. It is therefore important to preserve these tissues with minimal changes in morphology. The goal of this study is to investigate the difference between in vivo and ex vivo OCT imaging and the effect of different preservation solutions on image quality using the hamster cheek pouch. The hamster cheek pouch was chosen because of its easy access and because it is a well established model for carcinogenesis and cancer progression. The advent of the ultrahigh resolution OCT imaging technology is important for this study because it enables changes in tissue morphology that may have been difficult to resolve with standard resolution OCT imaging to be clearly visualized. OCT imaging was performed using an ultrahigh resolution OCT imaging system [3]. An axial resolution of 2 µm and a transverse resolution of 5 µm were employed. Several different preservation solutions were evaluated including: saline at low temperature, saline at room temperature, phosphate buffered sucrose (PBS140), University of Wisconsin solution (UW), and 10% formalin. Phosphate buffered sucrose and University of Wisconsin solution are common tissue preservation solutions, while formalin is commonly used for tissue fixation before histological processing. Following the anesthetization of the animal, the imaging areas in the cheek pouch were marked with india ink. For each imaging site, a 0.75 mm (axial) by 1 mm (transverse) OCT image was obtained. Each image consisted of 2000 axial pixels and 1000 transverse pixels to ensure that the pixel density was sufficient for high resolution imaging. The multiple imaging sites were then resected and the specimens placed in room temperature saline, chilled saline, PBS140, UW or 10% formalin. The marked areas in the different preservation solutions were imaged at 30, 50, and 70 minutes post mortem. The image quality, details, and contrast for each solution were then compared. At the conclusion of the experiments, the tissue was fixed in formalin and histologically processed. Figure 22 shows an example of OCT imaging data, which is a compilation of the in vivo images, the effects of different preservation solutions at 70 minutes postmortem, and the corresponding histology. The top row (a-e) represents in vivo OCT images of the hamster cheek pouch tissue taken at different imaging sites. The middle row (f-j) represents ex vivo OCT images of the same imaging site as the top row taken at 70 minutes postmortem. These areas were imaged ex vivo while preserved in a saline ice bath (f), room temperature saline (g), phosphate buffered sucrose 140 (h), University of Wisconsin solution (i) and 10% formalin (j). All the OCT images were collected at a resolution of 2 µm x 5 µm at 800 nm central wavelength. The bottom row represents the corresponding histology stained with Trichrome. The histology slides are viewed at 40x and were scaled to match the image size. 27-21 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Figure 22. In vivo (a-e) and 70 min. postmortem ex vivo (f-j) ultrahigh resolution OCT images of the hamster cheek pouch tissue under different preservation solutions and their corresponding histologic cross-sections stained with Trichrome (k-o). Evaluation of these images indicates that most changes in optical properties and OCT contrast in tissue occur within minutes after excision for all preservation solutions but formalin. The contrast between the epithelial cells and the stratum corneum as well as the underlying connective tissue is rapidly lost in almost all solutions, with the exception of formalin. The low backscattering nature of the dense connective tissue is not preserved. This could be the result of leakage of intracellular material from the cells into the extracellular space or expansion of the spaces between the normally dense collagen fibers. In formalin, the reflection of the two layers above and below the epithelial cells increases in intensity. Modification of the backscattering intensity of the lower, looser collagen fibers is also visible ex vivo. The epithelial layer, which is often the region of interest in neoplastic imaging, appears to be very sensitive to ex vivo preservation conditions. Although formalin is used routinely for histological processing, it has long been considered a poor option for tissue preservation in optical imaging studies because it changes the fluorescent and optical properties of tissue. However, our study indicates that formalin may actually be a better preservative for ex vivo, morphological OCT imaging. If the goal of the study is to image and evaluate variations in tissue morphology, then fixation may change the contrast of some compartments, but it also appears to better maintain the architectural features of interest. Intravascular Imaging using Optical Coherence Tomography Sponsors National Institute of Health Grant NIH-1-R01-HL63953-01 Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-5-R01-CA75289-04 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Pei-Lin Hsiung, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Professor Mark Brezinski, and Professor James G. Fujimoto 27-22 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 OCT has been shown to be an effective intravascular imaging technique for the discrimination of coronary plaques and assessment of interventional procedures such as arterial stent placement [25, 26]. Guided placement of coronary stents have greatly improved the outcome of interventional intravascular procedures and constitutes the majority of percutaneous interventions [27, 28]. Current intracoronary stent procedures require a secondary ultrasound (IVUS) catheter to be introduced after removal of a balloon catheter in order to assess stent deployment efficacy. In addition to increased operational time which incurs higher risk to the patient, IVUS resolution is limited to only ~80 µm at an operational frequency of 40 MHz. OCT has several advantages for intravascular imaging. High resolution OCT imaging with discrimination of sub-cellular features has been demonstrated indicating a resolution capability down to 10-20 µm. Further because OCT is a fiber-optically based imaging technique, extremely small and compact probes have been demonstrated with outer diameters of <500 µm [13]. The simple design and components of OCT catheters make them relatively inexpensive as well as highly compact when compared with IVUS catheters and ultrasound machines. Finally OCT can be performed at 4-8 frames per second allowing near real-time imaging during coronary procedures. In vitro imaging of human coronary arteries was performed using both OCT and IVUS. Figure 23 shows an OCT image of a coronary artery and the corresponding histology. Consistent with previous work, OCT images of plaque correlate well with histopathology. Most significantly, OCT images demonstrate relatively good contrast among the layers of the vessel wall. This allows delineation of both the tunica intima and tunica media within the artery. Histology confirms the presence and morphology of the intimal hyperplasia seen in the OCT image of this vessel. Figure 23. A comparison of OCT and histology of a human coronary artery. (a) Discrimination of tunicae intima, media, and adventitia can be seen in the OCT image. (b) Hematoxylin-eosinstained sections of the artery verify intimal hyperplasia layers seen in the OCT image. In addition to in vitro arterial imaging, in vivo OCT procedures were done in New Zealand white rabbits. Images were recorded at 4 frames/second with some slight motion artifacts noted due to the slower scan speed. Figure 24 (a) shows an aorta image after a manual 2-3 ml/sec saline flush. In the image, the media and supporting structures are well defined. Backscattering intensity was high in the media and low in the adventitia which consists primarily of loose connective tissue. Histology was used to confirm structural morphology seen in the OCT image (Figure 24 (b)). 27-23 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 Figure 24. Aorta image after saline flush and corresponding histology. (a) The media surrounding the supporting structures are clearly defined. High backscattering is apparent in the media “M” and low in the adventitia which consists of mainly loose connective tissue. A structure consistent with the inferior vena cava was also apparent and is imaged through the wall of the aorta. (b) Corresponding histology to confirm tissue identification. Scale bar represents 500 µm. The high resolution of OCT allows imaging to be performed near the resolution of histopathology, offering better discrimination and delineation of arterial structure and morphology. OCT has demonstrated the potential to have a significant impact on both the identification of high risk coronary plaques and in guidance of interventional arterial procedures. Orthopedic Imaging and Detection of Early Osteoarthritic Changes Sponsors National Institute of Health Grant NIH-1-R01-HL63953-01 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-5-R01-CA75289-04 National Institute of Health Grant NIH-1-R01-AR44812-02 National Institute of Health Grant NIH-1-R01-EY131780-01 Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Pei-Lin Hsuing, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Nirlep Patel, Karl Schneider, Dr. Scott Martin (Brigham and Women’s Hospital), Professor Mark Brezinski, and Professor James G. Fujimoto Osteoarthritis (OA) is the leading cause of chronic disability in developed countries, symptomatically affecting about 14% of the adult population in the United States alone [29]. The earliest pathological changes are collagen disorganization, an increase in the water content, and alteration in glycosaminoglycans (GAGS). Among the later changes are cartilage loss (thinning 27-24 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 effect), fibrillation, and surface erosion [30]. Until recently, osteoarthritis has been accepted as an inescapable consequence of aging. There is now strong evidence that osteoarthritic progression can be slowed or modified through surgical and/or pharmacological intervention [31, 32]. Current imaging technologies however have only a limited ability to monitor changes in articular cartilage. Available techniques such as plain film and magnetic resonance imaging (MRI) have resolutions of no better than 10 mm, which limits their use in monitoring cartilage changes [29, 33-35]. Although arthroscopy is widely used in diagnosis of joint disorders [36], it provides magnified views of only the articular surface. Thus, the pathologic changes under the articular cartilage surface cannot be visualized. A diagnostic imaging technique capable of high-resolution imaging of articular cartilage in vivo would be highly valuable to detect disease, to follow its progression and to monitor therapeutic effectiveness. In this study, human imaging of normal and osteoarthritic cartilage was demonstrated with optical coherence tomography using both a hand held probe and an approximately 1 mm arthroscopic imaging probe. Imaging was performed of human cartilage during at total of 8 total knee replacements in real time at 4-8 frames per second. Images compared favorably to histopathology and clear distinctions were noted between diseased and normal regions. In addition to identifying cartilage thinning, changes in back-reflection intensity and polarization were noted in diseased regions. Figure 25 demonstrates the cross-sectional OCT image of an osteoarthritic joint obtained with the portable high speed OCT system. The pathologic portion of the cartilage is severely degenerated; the junction between the normal and pathologic cartilage can be resolved. The figures show a representative OCT image of the arthritic knee cartilage transitional zone from articular cartilage to full thickness cartilage loss and to bone (black arrows). The region of the subchondral plate is demonstrated by red arrows. A corresponding trichrome blue histologic section through the same image plane as the OCT image is also shown (right). OCT Trichrome-Blue 500um Figure 25. (left) Representative intraoperative OCT image of human osteoarthritic articular cartilage taken with a hand-held imaging probe during total knee replacement surgery. (right) Corresponding histological section with trichrome-blue staining. OCT imaging was also performed in human knees with an OCT arthroscope. Again, highresolution structural assessments of articular cartilage could be obtained, including the demonstration of polarization sensitivity. Imaging was performed using a 1 mm diameter optical imaging catheter. The catheter is shown schematically in Figure 6 and consists of a single mode fiber in a rotating speedometer cable with a distal lens and microprism encased in a transparent housing [3]. Imaging was performed in vitro on an intact human knee obtained from autopsy to examine the feasibility of performing OCT through a cannula during arthroscopy. Figure 26 shows examples of images obtained with the OCT imaging probe. The images show the boundary of cartilage and bone (left) and a ligament (right). The ligament has a high birefringence due to the organization of collagen. The multiple striped features in the OCT image are the result of polarization birefringence. 27-25 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 500 um 500 um Figure 26. Representative OCT images taken using an OCT imaging catheter introduced through a cannula in an intact knee in vitro. The image on the left shows the junction between cartilage and bone. The image on the right shows a ligament which exhibits strong birefringence. These studies demonstrate that OCT imaging can be performed in an intact knee joint in vitro using an OCT imaging catheter introduced through a cannula in a minimally invasive procedure. This procedure is an important step toward performing OCT imaging in the clinic. It is clear that OCT represents a promising new technology for monitoring changes in articular cartilage, both in an open surgical field and through an arthroscope. Prostate Cancer Detection and Imaging Neoplastic Changes Sponsors Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 Air Force Office of Scientific Research Grant F49620-98-01-0084 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-5-R01-CA75289-04 National Institute of Health Grant NIH-1-R01-EY131780-01 Binney Radiation Oncology Fund Project Staff Aaron Aguirre, Tony Ko, Pei-Lin Hsuing, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Christopher Kroeger, Dr. Xingde Li, Karl Schneider, Dr. Anthony D’Amico, Dr. Michael Weinstein, Dr. Jerome Richie (Brigham and Women’s Hospital), Dr. Phillip Kantoff (Dana Farber), and Professor James G. Fujimoto The lifetime risk in Western society of developing histologic evidence of prostate cancer has been estimated to be 30%, with the risk of developing clinical disease 10% and the probability of mortality due to the disease roughly 3% [37]. Core biopsy is the standard method for diagnosing prostate cancer. However biopsy techniques often have sampling errors, which lead to false negatives. There has therefore been significant interest in novel techniques to improve the diagnostic accuracy in prostate cancer. Ex vivo, ultrahigh resolution OCT imaging studies suggest that differentiation of glandular architectural morphology associated with prostate cancer is feasible [38]. Devices such as OCT needles have been demonstrated which enable imaging in solid tissues or organs [13]. A technology capable of imaging the prostate in vivo at high resolution could be integrated with core biopsy to reduce sampling error. 27-26 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 We have performed preliminary imaging studies to investigate the feasibility of OCT for imaging prostate pathology in vivo. Imaging intraoperatively was chosen to eliminate the potential effects of tissue degradation on contrast or image quality as well as to investigate the feasibility of using OCT in an intraoperative setting. Informed consent was obtained from patients with prostate cancer scheduled to undergo nerve-sparing radical prostatectomy. The patient selection criterion was clinical stage T1-T4 adenocarcinoma of the prostate as evident on core biopsy. This patient population was selected because of the likelihood of a large tumor that might be visible with intraoperative OCT imaging through the prostatic capsule. To date, imaging has been performed on 2 patients with clinical stage T1-T4 adenocarcinoma of the prostate undergoing nerve-sparing radical prostatectomy. Images were taken of the posterior surface of the prostate after the urethra was divided and the prostate is retracted. Figure 27 shows an example intraoperative OCT image of the right posterior peripheral zone of the prostate with corresponding histology. Striations in the fibromuscular stroma are clearly visible in both the OCT image and histology. 250 µm 250 µm Figure 27. Representative intraoperative OCT image of normal prostate in vivo. Striations in the fibromuscular stroma are visible in both the OCT image and in the corresponding histologic photomicrograph. A 250 µm B 250 µm C 250 µm Figure 28. Representative in vivo OCT images of (a) large vein just underneath the prostate capsule, (b) a large gland, (c) lipid layer overlying normal prostate tissue. 27-27 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 A variety of structures in the normal prostate can also be identified with OCT. Figure 28 shows examples of a large vein underlying the prostate capsule, a large benign gland, and a thin layer of lipid-filled structures overlying normal prostatic tissue. Imaging was also performed ex vivo in the pathology laboratory. Imaging in the pathology laboratory immediately after surgical excision enables accurate registration of OCT images with histology as well as a comparison of in vivo versus ex vivo imaging. In order to preserve diagnostic integrity, specimens often cannot be removed from the hospital, and thus the capability of imaging in the pathology lab setting is especially important as a research tool. OCT images of the prostate taken ex vivo showed no significant degradation in contrast or image quality when imaging is performed immediately after excision of the specimen. These studies demonstrate the feasibility of using OCT to image human prostatic tissue in vivo. Additional studies are required to evaluate the ability of OCT to differentiate prostate cancer. Advances in solid-state laser and nonlinear fiber technology have allowed development of ultrahigh resolution OCT which enables 1-3 µm resolution imaging, and spectroscopicallyresolved OCT using broadband light sources also promises to improve tissue differentiation and image contrast. The ability to visualize tissue pathology in situ and in real time promises to improve both diagnosis and therapy of prostate carcinoma. These studies were conducted in collaboration with Dr. Mike Weinstein and Dr. Anthony D’Amico at Brigham and Women’s Hospital and Dr. Phillip Kantoff at the Dana Farber Cancer Center. Surgical Guidance using Optical Coherence Tomography Sponsors Air Force Office of Scientific Research (MFEL) Grant F49620-01-1-0186 Air Force Office of Scientific Research Grant F49620-98-01-0084 National Institute of Health Grant NIH-2-R01 EY11289-15 National Institute of Health Grant NIH-5-R01-CA75289-04 National Institute of Health Grant NIH-1-R01-AR44812-02 National Institute of Health Grant NIH-1-R01-HL63953-01 Project Staff Aaron Aguirre, Ravi Ghanta, Tony Ko, Pei-Lin Hsiung, Paul Herz, Dr. Stephane Bourquin, Dr. Christian Chudoba, Dr. Wolfgang Drexler, Dr. Ingmar Hartl, Dr. Christopher Kroeger, Dr. Ping Xue, Nirlep Patel, Karl Schneider, Dr. Anthony D’Amico, Dr. Michael Weinstein, Dr. Jerome Richie (Brigham and Women’s Hospital), Dr. Phillip Kantoff (Dana Farber), Professor Mark Brezinski, and Professor James G. Fujimoto Optical coherence tomography can image microstructure in tissue in situ and in real time with image resolutions in the 1-10 µm range [1, 3, 39]. Previous studies have demonstrated that changes in tissue architectural morphology associated with neoplasia can be identified [39-41], and recent advances such as ultrahigh resolution and spectroscopic OCT promise to further improve the differentiation of tissue pathology [3, 9]. The development of intraoperative OCT enables pathology to be visualized in situ and in real time in tissues that were previously inaccessible in vivo. Combining Intraoperative studies with imaging in the pathology laboratory 27-28 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 immediately after surgical excision enables accurate registration of OCT images with histology as well as a comparison of in vivo versus ex vivo imaging. In order to preserve diagnostic integrity, specimens often cannot be removed from the hospital, and thus the capability of imaging in the pathology lab setting is especially important as a research tool. Techniques that can visualize microscopic disease in situ and in real time promise to improve diagnostic accuracy. The ability to visualize tissue structures intraoperatively should also facilitate the guidance of microsurgical procedures [42]. In order to perform intraoperative imaging, an OCT system must be ergonomically well-designed and robust to function under the tight confines and time constraints of the surgical suite. An image delivery device that is sterilizable using standard hospital sterilization protocols is also required. We have developed a sterilizable hand-held surgical probe used with a portable OCT system to image in the surgical suite and pathology laboratory. The imaging probe has a 2 cm working distance which allows the surgeon to maneuver the beam within the tight confines of the surgical field. Figure 29 shows a schematic of the probe and a photograph of the operative field during radical prostatectomy. A low power green aiming beam allows the surgeon to localize the imaging area and facilitate marking for correlation with histology. The probe has a stainless steel outer tube with a transparent window which can be sterilized using standard hospital sterilization procedures and slipped over the distal end of the probe prior to imaging. The proximal end of the probe including cables was enclosed in a sterile surgical camera bag. The probe produced a focused spot with an 18 µm transverse resolution. The portable OCT imaging system used a semiconductor amplifier (AFC) low coherence light source operating at 1300 nm wavelength having an axial resolution of 15 µm in air. Images of 500 to 250 transverse pixels by 700 axial pixels could be obtained at 4-8 frames per second, respectively, with 95 dB sensitivity using a less than 6 mW incident power imaging beam. Urethra l f2 f2 + f3 f3 f4 Prosta f1 Semin al fiber Figure 29. (left) Schematic OCT hand-held probe and (right) picture of the surgical field. The probe uses a galvanometer controlled mirror and Hopkin’s lens relay system to acquire OCT images over a 3 mm field of view. A sterile stainless steel outer sheath covers the distal end of the probe. A urethral catheter aids intraoperative manipulation of the prostate. We have performed intraoperative imaging studies of patients with prostate cancer undergoing radical prostatectomy and patients with osteoarthritis undergoing open field surgery. Studies were performed to investigate the feasibility of using OCT for imaging these pathologies in vivo as well as to investigate OCT intraoperative imaging in a more general context. Imaging in the pathology laboratory has also been performed immediately after surgical excision to enable accurate registration of OCT images with histology as well as a comparison of in vivo versus ex vivo imaging. Preliminary results demonstrate the feasibility of intraoperative OCT imaging in human subjects as well as imaging in a pathology laboratory environment. Recently we have designed a version of the probe that uses two orthogonally positioned galvanometer mirrors to enable scanning of the OCT beam in the en face plane. This has enabled us to use the probe for in vivo investigations of optical coherence microscopy, a technique that combines standard OCT imaging with confocal microscopy. Extension of these technologies into the intraoperative setting and the 27-29 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 pathology laboratory should enable comprehensive imaging studies. The ability to visualize tissue pathology in situ and in real time promises to improve both diagnosis and therapy. Publications U. Morgner, W. Drexler, F.X. Kärtner, X. D. Li, C. Pitris, E. P. Ippen, J. G. Fujimoto, “Spectroscopic optical coherence tomography,” Optics Letters 25, 111-113, January 15, 2000. J. G. Fujimoto, C. Pitris, S. Boppart, and M. Brezinski, “Optical coherence tomography, an emerging technology for biomedical imaging and optical biopsy,” Neoplasia 2, 9-25, January 2000. C. Pitris, C. Jesser, S. A. Boppart, D. Stamper, M. E. Brezinski, and J. G. Fujimoto, “Feasibility of optical coherence tomography for high resolution imaging of human gastrointestinal tract malignancies,” J. Gastroenterology 35, 87-92, February 2000. P. Patwari, N. J. Weissman, S. A. Boppart, C. A. Jesser, D. Stamper, J. G. Fujimoto, and M. E. Brezinski, “Assessment of coronary plaque with optical coherence tomography and high frequency ultrasound,” Journal of the American College of Cardiology, 85, 641-644, March 2000. S. A. Boppart, J. M. Herrmann, and J. G. Fujimoto, “Optical coherence tomography: Applications in surgical diagnosis, guidance and intervention,” Medical Imaging International 10, 14-20, MarchApril 2000. A. V. D’Amico, M. Weinstein, X. Li, J. P. Richie, and J. G. Fujimoto, “Optical coherence tomography as a method for identifying benign and malignant microscopic structures in the prostate gland,” Urology 55, 783-787, 2000. J. Van Dam and J. G. Fujimoto, “Imaging beyond the endoscope,” Gastrointestinal Endoscopy 51, Part 1, 512-516, 2000. W. Drexler, U. Morgner, R. K. Ghanta, J. S. Schuman, F. X. Kartner, M. R. Hee, E. P. Ippen, and J. G. Fujimoto, “New technology for ultrahigh resolution optical coherence tomography of the retina,” in The Shape of Glaucoma, Quantitative Neural Imaging Techniques, H. G. Lemij and J. S. Schuman, Eds., pp. 75-104, Kugler Publications, The Hague, Netherlands, 2000. X. D. Li, C. Chudoba, T. Ko, C. Pitris, and J.G. Fujimoto, “Imaging needle for optical coherence tomography,” Optics Letters 25, October 2000. X. D. Li, S. A. Boppart, J. Van Dam, H. Mashimo, M. Mutinga, W. Drexler, M. Klein, C. Pitris, M. L. Krinsky, M. E. Brezinski, and J. G. Fujimoto, “Optical coherence tomography: advanced technology for the endoscopic imaging of Barrett's Esophagus,” Endoscopy 32, 921-30, December 2000. W. Drexler, U. Morgner, R. K. Ghanta, F. X. Kärtner, J. S. Schuman, J.G. Fujimoto, “Ultrahigh resolution ophthalmic optical coherence tomography,” Nature Medicine 7, 502-507, April 2001. C. Chudoba, J. G. Fujimoto, E. P. Ippen, H. A. Haus, U. Morgner, F. X. Kaertner, V. Scheuer, G. Angelow, and T. Tschudi, “All-solid-state Cr:forsterite laser generating 14 fs pulses at 1.3 mm,” Optics Letters 26, 292-294, March 2001. I. Hartl, X.D. Li, C. Chudoba, R. Ghanta, T. Ko, J.G. Fujimoto, J. K. Ranka, R. S. Windeler, and A. J. Stentz, “Ultrahigh resolution optical coherence tomography using continuum generation in an air-silica microstructure optical fiber,” Optics Letters 26, 608-610, May 2001. 27-30 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 C. Pitris, K. T. Saunders, J. G. Fujimoto, and M. E. Brezinski, “High resolution imaging of the middle ear with optical coherence tomography, a feasibility study,” Archives of Otolaryngology Head and Neck Surgery, 127, 637-42, June 2001. W. Drexler, D. Stamper, C. Jesser, X. D. Li, C. Pitris, K. Saunders, S. Martin, M. B. Lodge, J. G. Fujimoto, and M. E. Brezinski, “Correlation of collagen organization with polarization sensitive imaging of in vitro cartilage: Implications for osteoarthritis,” Journal of Rheumatology, 28, 1311-8, June 2001. S. A. Boppart, J. M. Herrmann, C. Pitris, D. L. Stamper, M. E. Brezinski, and J. G. Fujimoto, “Real-time optical coherence tomography for minimally-invasive imaging of prostate ablation,” Computer Aided Surgery 6, 94-103, 2001. X. Li, T.H. Ko, J.G. Fujimoto, “Intraluminal fiber-optic Doppler imaging catheter for structural and functional optical coherence tomography,” Optics Letters, 26, 1906-09, December 2001. J.G. Fujimoto, “Optical coherence tomography,” (C. R. Acad. Sci. Paris, Serie IV) Applied Physics, 1099-1111, 2001. X. Li, S. Martin, C. Pitris, R. Ghanta, C. Jesser, M. Spectro, J. G. Fujimoto, and M. E. Brezinski, “High-resolution imaging of osteoarthritic cartilage,” Journal of Rheumatology, submitted for publication. X. D. Li, H. Gold, N. Weissman, K. Saunders, C. Pitris, J. G. Fujimoto, and M. E. Brezinski, “Optical Coherence tomography guided stent deployment: a comparison with IVUS in an in vivo rabbit model,” Journal of the American College Cardiology, submitted for publication. Abstracts and Conference Presentations I. Hartl, X. Li, C. Chudoba, R. K. Ghanta, T. H. Ko, J. G. Fujimoto, J. K. Ranka, R. S. Windeler, and A. J. Stentz, “Ultrahigh resolution OCT using continuum generation in an air-silica microstructure optical fiber,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4251-07. X. Li, C. Chudoba, T. H. Ko, C. Pitris, R. K. Ghanta, and J. G. Fujimoto, “Imaging solid tissues with an OCT imaging needle,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4251-09. P. Hsiung, X. Li, I. Hartl, C. Chudoba, T. H. Ko, R. K. Ghanta, and J. G. Fujimoto, “High-speed path length scanning for optical coherence tomography,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4251-15. C. Pitris, T. H. Ko, W. Drexler, R. K. Ghanta, X. Li, C. Chudoba, I. Hartl, J. G. Fujimoto, and M. E. Weinstein, “Ultrahigh resolution in-vivo OCT imaging and tissue preservation,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4251-29. W. Drexler, R. K. Ghanta, J. S. Schuman, T. H. Ko, U. Morgner, F. X. Kärtner, and J. G. Fujimoto, “In-vivo optical biopsy of the human retina using optical coherence tomography,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4251-32. P. Hsiung, C. Pitris, X. Li, A. Goodman, R. K. Ghanta, T. H. Ko, C. Chudoba, I. Hartl, W. Drexler, M. E. Brezinski, and J. G. Fujimoto, “In-vivo cervical imaging with an integrated optical coherence tomography colposcope,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4254-31. 27-31 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 X. Li, S. A. Boppart, J. Van Dam, H. Mashimo, M. Mitinga, W. Drexler, M. Klein, C. Pitris, M. L. Krinsky, M. E. Brezinski, and J. G. Fujimoto, “Imaging Barrett’s esophagus with optical coherence tomography,” International Biomedical Optics Symposium, BIOS’2001, San Jose, CA, January 20-26, 2001, paper 4254-32. T.H. Ko, R.K. Ghanta, I. Hartl, W. Drexler, J.G. Fujimoto, “Ultrahigh resolution optical coherence tomography for quantitative topographic mapping of retinal and intraretinal architectural morphology,” Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, FL, April 29-May 4, 2001, paper 4251-B270. I. Hartl, T. Ko, R.K. Ghanta, W. Drexler, A. Clermont, S.E. Bursell, J.G. Fujimoto, “In vivo ultrahigh resolution optical coherence tomography for the quantification of retinal structure in normal and transgenic mice,” Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, FL, April 29-May 4, 2001, paper 4252-B271. W. Drexler, I. Hartl, T. Ko, R.K. Ghanta, J.S. Shuman, S.E. Bursell, J.G. Fujimoto, “Ultrahigh resolution imaging of retinal diseases with optical coherence tomography,” Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, FL, April 29-May 4, 2001, paper 636. P. Hsiung, C. Chudoba, C. Pitris, X. Li, T. Ko, and J. G. Fujimoto, “In vivo colposcopie imaging of neoplastic tissue using optical coherence tomography,” Conference on Laser and Electro-Optics, CLEO’01, Baltimore, MD, May 6-11, 2001, paper CTuY2. T. Ko, C. Pitris, I. Hartl, R. Ghanta, C. Chudoba, X. D. Li, W. Drexler, J. G. Fujimoto, and M. Weinstein, “Ultrahigh resolution in vivo versus ex vivo OCT imaging and tissue preservation,” Conference on Laser and Electro-Optics, CLEO’01, Baltimore, MD, May 6-11, 2001, paper CTuY2. I. Hartl, C. Chudoba, T. Ko, X. D. Li, J. G. Fujimoto, M. Brezinski, and R. S. Windeler, “Imaging water absorption with spectroscopic optical coherence tomography,” Conference on Laser and Electro-Optics, CLEO’01, Baltimore, MD, May 6-11, 2001, paper CWE2. P. Hsiung, X. D. Li, I. Hartl, T. Ko, C. Chudoba, and J. G. Fujimoto, “High-speed path length scanning using a Herriott cell delay line,” Conference on Laser and Electro-Optics, CLEO’01, Baltimore, MD, May 6-11, 2001, poster CWA62. J. G. Fujimoto, “Biomedical imaging and optical biopsy using optical coherence tomography,” 24th Annual Meeting of the Japan Society of Bronchology, May 24-25, 2001, Chiba, Japan, invited talk. J. G. Fujimoto, “Optical coherence tomography: current status and future prospects,” Schepens International Society Meeting, 2001 and Beyond- A Vetreoretinal Odyssey, Las Vegas, NV, June 6-9, 2001, invited talk. I. Hartl, C. Chudoba, T. Ko, X. D. Li and J. G. Fujimoto, R. S. Windeler, “Optical coherence tomography using continuum generation in an air-silica microstructure optical fiber,” European Conference on Biomedical Optics, ECBO, Munich, June 17-22, 2001, paper MH2. J. G. Fujimoto, I. Hartl, W. Drexler, C. Chudoba, T. Ko, X. Li, P. Hsiung, U. Morgner, and F. X. Kärtner, “Biomedical imaging using ultralight resolution optical coherence tomography,” XVII International Conference on Coherent and Nonlinear Optics (ICONO-2001), Minsk, Belarus, June 26 - July 1, 2001, paper 716, invited talk. 27-32 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 J. G. Fujimoto, “Optical biopsy using optical coherence tomography,” Engineering Foundation Conference, Advances In Optics For Biotechnology, Medicine And Surgery, Banff, Canada, July 22-27, 2001. J. G. Fujimoto, “Optical coherence tomography imaging: technology and applications,” International Congress on Analytical Sciences 2001, Tokyo, Japan, August 6-10, 2001, invited talk. X. Li, I. Hartl, C. Chudoba, W. Drexler, T. Ko, P. Hsiung, C. Pitris, R. Ghanta, F. X. Kärtner, U. Morgner, M. E. Brezinski, and J. G. Fujimoto, “Ultrahigh resolution and spectroscopic optical coherence tomography,” OSA Annual Meeting, ILS-XVII: 17th Interdisciplinary Laser Science Conference, Long Beach, CA, October 14-18, 2001, paper WV1, invited talk. J. G. Fujimoto, “Optical coherence tomography for structural and functional imaging,” Imaging in 2020, Jackson Hole, WY, September 30 – October 4, 2001, invited talk. W. Drexler, I. Hartl, T. H. Ko, R. K. Ghanta, H. Sattmann, B. Hermann, B. Povazay, K. K. Bizheva, J. S. Schuman, O. Findl, S. Bursell, M. Stur, A. F. Fercher, and J. G. Fujimoto, “Enhanced visualization of retinal pathologies using ultrahigh resolution optical coherence tomography,” Photonics West, SPIE, San Jose, CA, January 19-25, 2002, paper 4611-41. X. Li, T. H. Ko, and J. G. Fujimoto, “Intraluminal fiber optic Doppler imaging catheter for structural and functional optical coherence tomography,” Photonics West, SPIE, San Jose, CA, January 1925, 2002, paper 4619-22. T. H. Ko, I. Hartl, W. Drexler, R. K. Ghanta, and J. G. Fujimoto, “Ultrahigh resolution optical coherence tomography for quantitative topographic mapping of retinal and intraretinal architectural morphology,” Photonics West, SPIE, San Jose, CA, January 19-25, 2002, paper 4619-40. J. G. Fujimoto, I. Hartl, P. Xue, T. H. Ko, C. Chudoba, W. J. Wadsworth, T. A. Birks, and R. S. Windeler, “Supercontinuum generation in photonic crystal fibers as light sources for OCT,” Photonics West, SPIE, San Jose, CA, January 19-25, 2002, paper 4633-26, invited talk. References [1] [2] [3] [4] [5] [6] D. Huang, E. A. Swanson, C. P. Lin, J. S. Schuman, W. G. Stinson, W. Chang, M. R. Hee, T. Flotte, K. Gregory, C. A. Puliafito, and J. G. Fujimoto, “Optical coherence tomography,” Science, vol. 254, pp. 1178-1181, 1991. J. G. Fujimoto, C. Pitris, S. A. Boppart, and M. E. Brezinski, “Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy,” Neoplasia, vol. 2, pp. 9-25, 2000. W. Drexler, U. Morgner, F. X. Kärtner, C. Pitris, S. A. Boppart, X. D. Li, E. P. Ippen, and J. G. Fujimoto, “In vivo ultrahigh resolution optical coherence tomography,” Optics Letters, vol. 24, pp. 1221-1223, 1999. B. E. Bouma, G. J. Tearney, I. P. Bilinsky, and B. Golubovic, “Self phase modulated Kerrlens mode locked Cr:forsterite laser source for optical coherent tomography,” Optics Letters, vol. 21, pp. 1839-1841, 1996. J. K. Ranka, R. S. Windeler, and A. J. Stentz, “Visible continuum generation in air-silica microstructure optical fibers with anomalous dispersion at 800 nm,” Optics Letters, vol. 25, pp. 25-7, 2000. T. A. Birks, W. J. Wadsworth, and P. S. J. Russell, “Generation of an ultra-broad supercontinuum in tapered fibers (Post-deadline),” presented at Conference on Lasers and Electro-Optics (CLEO), San Francisco, CA, 2000. 27-33 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] I. Hartl, X. D. Li, C. Chudoba, R. K. Ghanta, T. H. Ko, J. G. Fujimoto, J. K. Ranka, and R. S. Windeler, “Ultrahigh-resolution optical coherence tomography using continuum generation in an air–silica microstructure optical fiber,” Optics Letters, vol. 26, pp. 608610, 2001. M. D. Kulkarni and J. A. Izatt, “Spectroscopic optical coherence tomography,” CLEO '96. Summaries of Papers Presented at the Conference on Lasers and Electro Optics, vol. 9, pp. 59-60, 1996. U. Morgner, W. Drexler, X. Li, F. X. Kaertner, C. Pitris, S. A. Boppart, E. P. Ippen, and J. G. Fujimoto, “Spectroscopic optical coherence tomography,” Optics Letters, vol. 25, pp. 111-113, 2000. J. M. Schmitt, S. H. Xiang, and K. M. Yung, “Differential absorption imaging with optical coherence tomography,” Journal of the Optical Society of America A - Optics Image Science and Vision, vol. 15, pp. 2288-2296, 1998. U. S. Sathyam, J. B. W. Colston, L. B. D. Silva, and M. J. Everett, “Evaluation of optical coherence quantitation of analytes in turbid media by use of two wavelengths,” Applied Optics, vol. 38, pp. 2097-2104, 1999. X. Li, T. H. Ko, and J. G. Fujimoto, “Intraluminal fiber-optic Doppler imaging catheter for structural and functional optical coherence tomography,” Optics Letters, vol. 26, pp. 1906-1908, 2001. X. D. Li, C. Chudoba, T. Ko, C. Pitris, and J. G. Fujimoto, “Imaging needle for optical coherence tomography,” Optics Letters, vol. 25, 2000. M. Rajadhyaksha, M. Grossman, D. Esterowitz, R. H. Webb, and R. R. Anderson, “In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast,” J Invest Dermatol, vol. 104, pp. 946-52, 1995. J. A. Izatt, M. R. Hee, G. M. Owen, E. A. Swanson, and J. G. Fujimoto, “Optical coherence microscopy in scattering media,” Optics Letters, vol. 19, pp. 590-592, 1994. V. Westphal, H. W. Wang, and J. A. Izatt, “Real-time in vivo optical coherence microscopy,” presented at CLEO, Baltimore, MD, 2001. G. J. Tearney, B. E. Bouma, and J. G. Fujimoto, “High-speed phase- and group-delay scanning with a grating-based phase control delay line,” Optics Letters, vol. 22, pp. 18111813, 1997. J. P. Heritage, A. M. Weiner, and R. N. Thurston, “Picosecond pulse shaping by spectral phase and amplitude manipulation,” Optics Letters, vol. 10, pp. 609-611, 1985. A. V. Zvyagin and D. D. Sampson, “Achromatic optical phase shifter-modulator,” Optics Letters, vol. 26, pp. 187-190, 2001. B. E. Lemoff and C. P. J. Barty, “Quintic phase limited, spatially uniform expansion and recompression of ultrashort optical pulses,” Optics Letters, vol. 18, pp. 1651-1653, 1993. M. R. Hee, C. A. Puliafito, J. S. Duker, E. Reichel, J. G. Coker, J. R. Wilkins, J. S. Schuman, E. A. Swanson, and J. G. Fujimoto, “Topography of diabetic macular edema with optical coherence tomography,” Ophthalmology, vol. 105, pp. 360-370, 1998. C. A. Puliafito, M. R. Hee, C. P. Lin, E. Reichel, J. S. Schuman, J. S. Duker, J. A. Izatt, E. A. Swanson, and J. G. Fujimoto, “Imaging of macular diseases with optical coherence tomography,” Ophthalmology, vol. 102, pp. 217-229, 1995. J. S. Schuman, T. Pedut-Kloizman, E. Hertzmark, M. R. Hee, J. R. Wilkins, J. G. Coker, C. A. Puliafito, J. G. Fujimoto, and E. A. Swanson, “Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography,” Ophthalmology, vol. 103, pp. 1889-1898, 1996. X. D. Li, S. A. Boppart, J. Van Dam, H. Mashimo, M. Mutinga, W. Drexler, M. Klein, C. Pitris, M. L. Krinsky, M. E. Brezinski, and J. G. Fujimoto, “Optical coherence tomography: advanced technology for the endoscopic imaging of Barrett's esophagus,” Endoscopy, vol. 32, pp. 921-30, 2000. P. Patwari, N. J. Weissman, S. A. Boppart, C. A. Jesser, D. Stamper, J. G. Fujimoto, and M. E. Brezinski, “Assessment of coronary plaque with optical coherence tomography and high frequency ultrasound,” Journal of the American College of Cardiology, vol. 85, pp. 641-644, 2000. 27-34 27 - Photonic Materials, Devices and Systems – Laser Medicine and Medical Imaging Group – 27 RLE Progress Report 144 [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] S. A. Boppart, J. M. Herrmann, and J. G. Fujimoto, “Optical coherence tomography: Applications in surgical diagnosis, guidance and intervention,” Medical Imaging International, vol. 10, pp. 14-20, 2000. R. A. Schatz, “Clinical experience with the Palmaz-Schatz coronary stent,” J Am Coll Cardiol, vol. 17, pp. 155B-159B, 1991. P. N. Ruygrok and P. W. Serruys, “Intracoronary stenting: from concept to custom,” Circulation, vol. 94, pp. 882-90, 1996. M. E. Adams and C. J. Wallace, “Quantitative imaging of osteoarthritis,” Seminars in Arthritis and Rheumatism, vol. 20, pp. 26-39, 1991. W. N. Kelley, Textbook of Rheumatology, vol. I,II, 5th ed. Philadelphia: W.B. Sanders, 1997. C. J. Lozada and R. D. Altman, “Chondroprotection in osteoarthritis,” Bull Rheum Dis, vol. 46, pp. 5-7, 1997. J. A. Buckwalter and H. J. Mankin, “Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation,” Instr Course Lect, vol. 47, pp. 487-504, 1998. W. P. Chan, P. Lang, M. P. Stevens, K. Sack, S. Majumdar, D. W. Stoller, C. Basch, and K. Genant, “Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity,” American Journal of Roentgenology, vol. 157, pp. 799806, 1991. J. D. Rubenstein, J. G. Li, S. Majumdar, and R. M. Henkelman, “Image resolution and signal-to-noise requirements for MR imaging of degenerative cartilage,” American Journal of Roentgenology, vol. 169, pp. 1089-96, 1997. D. Loeuille, P. Olivier, D. Mainard, P. Gillet, P. Netter, and A. Blum, “Review: Magnetic resonance imaging of normal and osteoarthritic cartilage,” Arthritis and Rheumatism, vol. 41, pp. 963-75, 1998. R. W. Ike, “Diagnostic arthroscopy,” Baillieres Clinical Rheumatology, vol. 10, pp. 495517, 1996. S. Kirby, T. J. Christmas, and M. Brawer, Prostate Cancer: Times Mirror International Publishers Ltd., 1996. A. V. D'Amico, M. Weinstein, X. Li, J. P. Richie, and J. Fujimoto, “Optical coherence tomography as a method for identifying benign and malignant microscopic structures in the prostate gland,” Urology, vol. 55, pp. 783-7, 2000. J. G. Fujimoto, M. E. Brezinski, G. J. Tearney, S. A. Boppart, B. Bouma, M. R. Hee, J. F. Southern, and E. A. Swanson, “Optical biopsy and imaging using optical coherence tomography,” Nature Medicine, vol. 1, pp. 970-972, 1995. C. Pitris, A. Goodman, S. A. Boppart, J. J. Libus, J. G. Fujimoto, and M. E. Brezinski, “High-resolution imaging of gynecologic neoplasms using optical coherence tomography,” Obstetrics and Gynecology, vol. 93, pp. 135-9, 1999. A. M. Sergeev, V. M. Gelikonov, G. V. Gelikonov, F. I. Feldchtein, R. V. Kuranov, N. D. Gladkova, N. M. Shakhova, L. B. Snopova, A. V. Shakov, I. A. Kuznetzova, A. N. Denisenko, V. V. Pochinko, Y. P. Chumakov, and O. S. Streltzova, “In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa,” Optics Express, vol. 1, pp. 432, 1997. S. A. Boppart, B. E. Bouma, C. Pitris, G. J. Tearney, J. F. Southern, M. E. Brezinski, and J. G. Fujimoto, “Intraoperative assessment of microsurgery with three-dimensional optical coherence tomography,” Radiology, vol. 208, pp. 81-86, 1998. 27-35