* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Warren, ST and Ashley, CT: Triplet repeat expansion mutations: The example of fragile X syndrome. Annual Review of Neuroscience 18:77-99 (1995).
Gene nomenclature wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Genetic engineering wikipedia , lookup
Medical genetics wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Epigenomics wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
X-inactivation wikipedia , lookup
Oncogenomics wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Genome (book) wikipedia , lookup
Down syndrome wikipedia , lookup
Designer baby wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Helitron (biology) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Frameshift mutation wikipedia , lookup
Microsatellite wikipedia , lookup
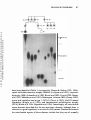
ANNUAL REVIEWS Further Quick links to online content Annu. Rev. Neurosci. 1995. 18:77-99 Copyright © 1995 by Annual Reviews inc. All rights reserved TRIPLET REPEAT EXPANSION Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. MUTATIONS: The Example of Fragile X Syndrome Stephen T. Warren and Claude T. Ashley, Jr. Howard Hughes Medical Institute and Departments of Biochemistry and Pediatrics, Emory University School of Medicine, Atlanta, Georgia 30322 KEY WORDS: FMRl gene, mental retardation. X chromosome. unstable DNA, DNA methylation INTRODUCTION Mental retardation represents a deficiency in intelligence, as measured by IQ, with limited adaptive behavior that is normally reflected in maturation, learn ing, or social adjustment (American Psychiatric Association 1987). Approxi mately 1 to 3% of the population, depending upon definitions of adaptive behavior, is mentally retarded (Popper 1988). The etiologies and determinants of mental retardation are diverse and include socioeconomic influences leading to extreme malnutrition and/or inadequate prenatal care; toxic insults, such as that leading to fetal alcohol syndrome; trauma and infection; and genetic factors (Popper 1988). At least 300 genetic disorders include mental retardation as part of the phenotype, and genetic components are considered important influences on related disorders such as attention deficit disorder and learning disability (Smith et al 1983, Biederman et al 1987, McKusick et al 1992). Since the early twentieth century a male predominance at all levels of mental retardation, ranging from 1.5 to 3 times the incidence in females, has been acknowledged (Penrose 1938). Although a variety of explanations have been put forth, including the probable ascertainment bias of mentally retarded males being more frequently institutionalized because of uncontrollable or violent behavior, there is good reason to believe X-linked loci contribute significantly to this gender inequity (Opitz 1986). Perhaps as many as 95 genes have been tentatively assigned to the X chromosome where mutations lead to mental retardation as at least part of the phenotype (Schwartz 1993). As the vast 77 Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 78 WARREN & ASHLEY majority of these mutations appear recessive, females, protected by an addi tional X chromosome, are rarely affected, and hence this leads to the excess male mental retardates. However, most of these disorders are exceedingly rare, and as single loci, none would be expected to appear frequently in the popu lation. Thus, an aggregate of all X-linked mutations involving mental retarda tion appeared responsible for gender differences until a single locus, that responsible for fragile X syndrome, became recognized as a clinical entity that eventually would account for nearly half of the male predominance of mental retardation (Opitz & Sutherland 1984) . HISTORICAL AND CLINICAL ASPECTS Fragile X syndrome, an X-linked dominant disorder with reduced penetrance, is the most frequent form of familial mental retardation, with a prevalence of 1 in 1500 males and 1 in 2500 females (Gustavson et al 1986, Webb et al 1986). It is the single most common etiology of X-linked mental retarda tion (Neri et al 1992, Brown & Jenkins 1992), and ranks second only to Down's syndrome, which is most often sporadic, as the most frequent genetic cause of mental retardation overall. First described by Martin & Bell (Martin & Bell 1943), the syndrome cosegregates with a marker X chromosome that is observed cytogenetically as a nonstaining constriction in the long arm of Figure 1 Partial metaphase spread from a patient with fragile X syndrome showing the iso chromatid gap (arrow) near the distal end of the X chromosome long arm, which is indicative of the fragile X site (From Warren & Nelson 1994. Used by permission). Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X SYNDROME 79 the X chromosome near the distal tip (Lubs 1969). Sutherland (1977) later identified this constriction as an inducible fragile site at Xq27.3 (Figure 1); it is induced in tissue culture by perturbation of folic acid or thymidine pools, which results in limited amounts of dTIP for DNA synthesis (Sutherland 1977, Glover 1981). Upon analysis by electron microscopy, the fragile site was described as an isochromatid gap at Xq27.3 (Harrison et al 1983). In fragile X males, levels of fragile site expression in metaphase spreads from peripheral lymphocytes range from 5-50% of X chromosomes analyzed, whereas the extent of expression in female heterozygotes is on the order of 1-30% (Sutherland 1977, Sutherland et al 1985). Levels of induced fragile site expression in metaphase spreads of normal controls have been shown to be less than 1% (Jenkins et al 1986). For many years, prenatal diagnosis and carrier testing relied upon scoring the fragile X marker in metaphase spreads, but recent advances in the field have rendered cytogenetic tests supplemen tary (Warren & Nelson 1994). The major clinical manifestations of fragile X syndrome in adult males most notably include: mental retardation, ranging from mild to severe; mild facial dysmorphia, characterized by long, narrow facies, large dysmorphic ears, and prominence of the jaw and forehead; and macroorchidism with testicular volumes greater than 25 ml (Hagerman 1991, Hagerman et al 1991). With the obvious exception of macroorchidism, all of the phenotypic features of fragile X described above may be exhibited by adult carrier females, although they tend to be less severe (Hagerman et al 1992). Additional clinical signs often contributing to the fragile X phenotype include connective tissue abnormali ties, such as joint hyperextensibility, pes planus, pectus excavatum, and mitral valve prolapse (Opitz et al 1984, Hagerman & Synhorst 1984, Hagerman et al 1984, Loehr et al 1986). The presence of autistic-like behaviors is frequently reported in fragile X males (and occasionally in female carriers). These be haviors include hand-flapping, rocking, or hand-biting (repetitive motor be haviors); gaze aversion; tactile defensiveness (decreased social and nonverbal communication skills); and repetitive speech patterns, perseveration, and echo lalia (dysfunctional verbal communication) (Brown et al 1982, Reiss et al 1986, Reiss & Freund 1992). Psychiatric disorders, such as chronic depression, schizotypal personality disorder, social avoidance, social anxiety, and with drawal, are reportedly more prevalent in fragile X carrier females than in multifeature-matched controls (Reiss et al 1988, Hagerman & Sobensky 1989, Freund et aI1993). Hyperactivity and attention deficit disorder (ADHD) appear to be common in fragile X males before puberty (Hagerman & Sobensky 1989), although it is unclear if these features are more frequent in fragile X syndrome than is normally encountered in general mental retardation (Fisch 1993). How ever, because of the subtlety of the fragile X phenotype prior to puberty, hyperactivity represents the presenting illness in many fragile X males (Hager- 80 WARREN & ASHLEY man & Sobensky 1989). Additionally, because the phenotype of fragile X syndrome is quite pleiotropic and difficult to diagnose de novo prior to puberty, detailed checklists involving precise anthropometric measurements have been devised (Butler et al 1991a, Butler et al 1991b, Butler et al 1992). Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. INHERITANCE PATTERN The inheritance pattern of fragile X syndrome is both complex and unusual for a sex-linked Mendelian trait. Although the inclusion of affected heterozy gous females as well as hemizygous males would suggest an X-linked domi nant mode of inheritance, the occurrence of apparently unaffected males who carry the fragile X chromosome has been well documented (Martin & Bell 1943, Dunn et al 1963, Howard-Peebles & Friedman 1985, Froster-Iskenius et al 1986). In analyses of a total of 206 fragile X pedigrees by both classical and com pl ex segregation analyses, Sherman et al (1984, 1985) reported a 20% deficit in affected males in the absence of evidence of any sporadic cases, a finding best explained by reduced penetrance in males of 80%. Given the fact that affected males rarely reproduced, these unusual nonpenetrant carrier males were termed transmitting males because they passed the fragile X chromosome to all of their obligate carrier daughters. Interestingly, nonpenetrant transmit ting males were also cytogenetically negative for fragile site expression in lymphocytes (Howard-Peebles & Friedman 1985, Froster-Iskenius et aI1986). Penetrance of mental impairment in females was also found to be reduced, with an overall penetrance of 35%, in the study by Sherman et al (1984), and disease expression was again found to be in direct correlation with cytogenetic expression of the fragile site. Thus, a model of an X-linked dominant gene with reduced penetrance was imposed (Sherman et al 1984, 1985). Sherman et al (1984, 1985) made a most peculiar observation that the risk of mental impairment associated with fragile X syndrome was a function of one's position within the pedigree. As mentioned, carrier males are both nonpenetrant and cytogenetically negative for fragile X syndrome. Interest ingly, Sherman et al (1984, 1985) found that the risk of mental impairment in obligate carrier daughters of carrier males approached 0%. Siblings of these males were also at relatively low risks of 9% and 5% in males and females, respectively. Offspring of normal carrier females, however, incurred risks of 38% (males) and 16% (females) for mental impairment, which corresponded to penetrances of 76% and 32%, while offspring of mentally impaired carrier females displayed penetrances of 100% (males) and 56% (females). Thus, disease expression was dependent upon passage of the fragile X through a female, which led some to hypothesize that a premutation in carrier males causing the fragile X chromosome to be more susceptible to alteration in female gametes might account for this observation (Pembrey et al 1985). This odd FRAGILE X SYNDROME 81 pattern of increasing risk with vertical transcention through a pedigree, referred to as the Sherman paradox, was unique in human genetics, and it was only after the molecular basis of fragile X syndrome was elucidated that this peculiar pattern of inheritance was fully understood. Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. IDENTIFICATION OF THE FRAGILE X MUTATION In 1991, the combined approaches of genetic mapping, physical mapping, and somatic cell genetics culminated in the positional cloning of the fragile X locus and the identification of the mutation responsible for fragile X syndrome. Prior to this time, the fragile X disorder had been mapped genetically through linkage analysis to the same interval as the Xq27 fragile site between the factor VIII (12 cM distal) and factor IX (5 cM proximal) loci, which narrowed the region of interest to approximately 20 megabases (Goodfellow et al 1985, Oberle et al 1986). Also localized to the region surrounding the fragile site were the enzymes HPRT (Xq26) (Pai et a11980) and G6PD (Xq28) (Filippi et aI1983). By using rodent cells deficient for both of these enzymes, Warren and cowork ers (Warren et al 1987, Warren et al 1990) successfully constructed somatic cell hybrids containing either exclusively proximal or exclusively distal por tions of the human fragile X chromosome. They did this by selection either for or against the human HPRT enzyme activity followed by histochemical staining for G6PD activity. Because it had previously been shown that the fragile X site was cytogenetically expressed in hybrid cells, chromosomal breakage at or near the fragile site was expected for most of the hybrids (Warren & Davidson 1984). This was confirmed through hybridization of known proximal and distal X chromosome markers to the obtained hybrids (Warren et aI1990). The availability of proximal and distal hybrids, as well as other X-breakpoint hybrids, allowed a number of new polymorphic loci to be mapped relative to the fragile site, which narrowed the gap to within 3 megabases (Suthers et al 1990, Hirst et al 1991, Suthers et al 1991, Rousseau et al 1991b). The newly identified battery of DNA markers bracketing the fragile X locus allowed isolation of yeast artificial chromosomes (YACs) with inserts spanning the fragile X hybrid breakpoints (Dietrich et al 1991, Heitz et al 1991, Verkerk et al 1991, Kremer et al 1991b). Two groups independently identified an aberrantly methylated CpG island within this region of DNA in fragile X patients. Because of the absence of normal cleavage by methylation-sensitive enzymes in fragile X cells, a band of anomalous mobility on pulsed-field gel electrophoresis were used to identify the abnormal CpG island (Bell et al 1991, Vincent et aI1991). Oberle et al (1991) identified a 9-kb DNA segment from the YAC 209G4 that contained the CpG island hypermethylated in fragile X patients and that detected an apparent insertion in fragile X patients on Southern blots. Yu Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 82 WARREN & ASHLEY et al ( 1991) and Verkerk et al (1991) concurrently yet independently identified a single restriction fragment of approximately 5 kb that contained both the proximal and distal fragile X hybrid breakpoints and also detected length variation in fragile X patients on Southern blots. Kremer et al ( 199 1a) further localized this instability to a single 1.0-kb Pst I fragment, pfxa2, that was sequenced and found to contain an unusual CGG trinucleotide repeat of 43 copies. By using the polymerase chain reaction (PCR) and primers flanking the repeat, Kremer et al (1991a) mapped the region of instability in pfxa2 to within the CGG trinucleotide repeat itself, thereby suggesting that the length variation observed in fragile X patients could result from length variation within the trinucleotide repeat. The translocation breakpoints of various hybrids were also mapped to within the CGG repeat, thus colocalizing the fragile site and the fragile X mutation to this novel trinucleotide repeat (Kremer et aI199 1a). In order to identify expressed sequences in the fragile X breakpoint cluster region, four cosmid subclones of the YAC 209G4, which spanned the region encompassing the erroneously methylated CpG island, were used as probes against a eDNA library from human fetal brain (Verkerk et al 1991). Two cDNA clones, BC22 and BCn, comprising a contig of 3765 nucleotides, were identified and the DNA sequence of each was obtained. Analysis of this sequence revealed a single major open reading frame that remained open at the 5' end and encoded a predicted polypeptide of 657 amino acids. The CGG repeat potentially displaying length variation in fragile X patients was con tained within the 5' portion of the open reading frame where it was initially thought to encode an uninterrupted stretch of 30 arginine residues. However, later studies demonstrate that the CGG repeat is confined to the 5' untranslated region and is, therefore, not translated (Ashley et a1 1993a) (see below). Upon northern analysis, a 4.8-kb transcript was detected in RNA from human brain and placenta, which suggested that approximately 1 kb of sequence remained to be determined. A zoo blot containing genomic DNA from a number of eukaryotes, including lower organisms such as nematode and yeast, displayed band(s) in lanes of all organisms except Drosophila when probed with BC22 under high stringency, thus indicating strong evolutionary conservation of this gene, referred to as FMRl (fragile X mental retardation 1), and implying functional importance of its protein product, FMRP. Initial data base searches at both the nucleotide and amino acid levels, however, produced no significant homologies with any previously reported sequences and provided no immedi ate clues as to the normal function of this predicted protein (see below). FRAGILE X TRINUCLEOTIDE REPEAT EXPANSION The delimitation of the region of DNA instability to within the CGG repeat suggested the possibility that length variation observed in fragile X carriers Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X SYNDROME 83 might result from the inherent instability of this reiterated trinucleotide repeat, which would be reminiscent of other tandemly repeated sequences such as the highly polymorphic dinucleotide repeats (Weber 1990). Indeed, analysis of the eGG repeat of FMRl across normal populations revealed that it was highly polymorphic, with repeat sizes ranging from 6-54 triplets and a mode of 30 (Fu et al 1991, Brown et al 1993, Jacobs et al 1993, Snow et al 1993). In analysis of repeat sizes in fragile X pedigrees, Fu et al (1991) discovered that individuals nonpenetrant for fragile X syndrome, carrier males and their obli gate carrier daughters, displayed eGG repeat lengths ranging from 54-200 repeats, whereas affected individuals exhibited repeat sizes in excess of 200 and often greater than 1000 repeats. This finding was in agreement with the previous reports of apparently smaller insertions in carriers and larger inser tions in affected individuals (Oberle et al 1991, Yu et al 1991, Kremer et al 1991a), which Oberle et al (1991) referred to as premutations and full muta tions, respectively, in order to relate the size of the insertion with penetrance of the disease. This correlation between repeat size and penetrance not only allowed resolution of the Sherman paradox (Fu et al 1991), but it also paved the way toward identification of other trinucleotide repeat expansion mutations by substantiating earlier claims (Harper 1989) that diseases displaying genetic anticipation, increasing severity, or decreasing age of onset of disease from one generation to the next might share a common form of mutation. In addition to establishing an association between repeat size and penetrance for fragile X syndrome, studies of the repeat length of FMRl also aided in defining the boundaries of meiotic instability. In the study by Fu et al (1991), all FMRl eGG repeats of 54 and greater displayed meiotic instability, and smearing of the full mutation bands on Southern blots suggested mitotic instability as well. In a recent analysis of 116 families referred for fragile X testing, a FMRl eGG repeat of 51 was shown to be meiotically stable through five generations, whereas a repeat of 57 displayed meiotic instability in the next generation (Snow et al 1993). Also in this study, a eGG repeat of only 61 in a carrier female expanded into the full mutation range in the following generation. However, another premutation allele of 90 in a different carrier female only expanded to 115 upon transmission to her offspring. Therefore, repeats of 54 and above clearly display meiotic instability and should be considered premutations; however, the expansion of premutation alleles into the full mutation range must be dependent upon other factors yet to be identified. METHYLATION OF THE FMRl GENE In addition to trinucleotide repeat expansion in fragile X patients, abnormal methylation of a CpG island located 250 base pairs upstream of the CGG repeat of FMRl was also found in individuals expressing the fragile X phe- Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 84 WARREN & ASHLEY notype (Bell et al 1991, Heitz et al 1991, Oberle et al 1991, Vincent et al 1991). Like expansion of the repeat, the extent of CpG-island methylation is directly correlated with disease expression because carrier males, which are unaffected, display unmethylated CpG islands. Individuals with the full mu tation, however, have fully methylated CpG islands (Oberle et al 1991). Un affected carrier females display methylation only on the inactive X chrom osome, while affected females tend to be skewed toward excess methylation (> 50%), although this is not always the case (Oberle et al 1991). The CGG repeat, which is itself rich in CpG dinucleotides, is also fully methylated in affected individuals with full mutations or at normal repeat copy number on the inactive X (Hansen et al 1992, Hornstra et al 1993). The identification of abnormal methylation associated with fragile X syndrome is in agreement with Laird's (1987) hypothesis, which states that fragile X syndrome results from persistence of an imprint that is due to failure of erasure of X inactivation in a carrier female. Methylation of the CpG island adjacent to the FMRI gene correlates with transcriptional silencing of FMRI and expression of the fragile X phenotype. In a study by Pieretti et al ".(1991), 16 of 20 affected males with expanded repeats in the full mutation range displayed fully methylated CpG islands and produced no FMRI transcript. Of the four affected males with FMRI expres sion, three were identified as mosaics for fully methylated full mutations and unmethylated premutations, and one was a methylation mosaic with a partially unmethylated full mutation. In a prenatal diagnosis of an affected fetus with a full mutation, Sutcliffe et al (1992) reported an absence of methylation in chorionic villus samples that correlated with normal levels of FMRI expres sion, while in fetal tissue a fully methylated CpG island was found but not FMRI message. Recently, four carrier males with repeat sizes between 200 and 400 but with unmethylated CpG islands and repeats were found to be intellectually and physically normal by observation (Loesch et aI1993). Con versely, two males exhibiting partially methylated CpG islands and CGG repeats between 200 and 300 copies also displayed mild clinical and physical manifestations of fragile X syndrome (McConkie-Rosell et al 1993). Although many carrier females have premutation alleles, a significant pro portion of the normal carrier females carry full mutations with expansions well beyond 200 repeats as well as the abnormal methylation of the FMRI gene. Rousseau et al (1991a) demonstrated that approximately half (53%) of women with full mutations are penetrant with IQ levels in the borderline and mentally retarded range. The other half presumably escape the effect of the full mutation because of X inactivation (lyonization) patterns. However, Taylor et al (1994) showed that the pattern of X inactivation, i.e the proportion of fragile X chromosomes active versus inactive, in peripheral lymphocytes of full mutation females was not a predictor of intellectual function. Thus, it is most reasonable Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X SYNDROME 85 to conclude that the protein encoded by FMRI is cell autonomous and that the proportion of cells in key regions of the brain with the fragile X chromosome in the active state (but still not expressing FMRI because of the abnormal methylation) are responsible for penetrance in females. Thus, the absence or reduction of FMRI expression as a function of meth ylation status of the upstream ep G island and the eGG repeat is thought to be the basis for fragile X syndrome. The fact that at least four fragile X patients lacking repeat expansion but possessing other mutations of the FMRI gene itself, three large deletions and one point mutation, have been identified sup ports this hypothesis (Gideon et al 1992, Wohrle et al 1992, De Boune et al 1993, Tarleton et aI1993). The role of repeat expansion in abnormal methyl ation, or vice versa, remains to be determined. DETECTION OF THE FRAGILE X MUTATION Both the expansion of the eGG repeat and the abnormal methylation of the FMRI gene are important diagnostic indicators. Methods have been developed to assess these mutational parameters in patients (reviewed by Warren & Nelson 1994). Detection can employ either Southern blotting (Figure 2a) or the polymerase chain reaction (Figure 2b). Southern blotting most commonly utilizes the digestion of genomic DNA with EcoR I and the methylation sensitive enzyme BssH II or Eag I. EcoR I digestion liberates a 5.2-kb fragment containing the promoter and first exon of FMRl, which includes the eGG repeat. Double digestion with BssH II or Eag I cleaves normal male DNA into 2.4-kb and 2.8-kb fragments; the latter fragment contains the trinucleotide repeat. The active X chromosome of normal females also displays this pattern, but the inactive normal X in a female is methylated, as is common for many X-linked loci, and is resistant to BssH II or Eag I digestion. Thus, normal females exhibit three bands as shown in lane two of Figure 2a. Premutation alleles exhibit a shift in the 2.8-kb band to a slightly higher molecular weight that reflects the increase in the CGO repeat to the 54-230 repeat range. Full mutations display a large, somewhat diffuse band that is generally not cleaved by BssH II or Eag I. These bands reveal the expansion of the eGG repeat into the range of many hundreds of triplets as well as the abnormal methylation of the FMRI gene. Females with full mutations display a similar pattern superimposed upon that of the normal X (in both the active and inactive states). Analysis by peR has allowed a much more precise estimation of repeat length, particularly in normal and premutation alleles (Fu et aI1991). However, amplification of full mutations is difficult, though not impossible (Pergolizzi et al 1992). As shown in Figure 2b, PCR amplification of the eGG repeat by using flanking primers of unique sequence demonstrates the remarkable insta- Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 86 Figure 2 WARREN & ASHLEY Molecular detection of the FMRl gene. (A) Southern blot of genomic DNA digested with EcoRI and BssHII, resolved through agarose, transferred to membrane, and probed with radiolabeled FMRl gene fragment pE5.1 (Verkerk et al 1991). (8) peR analysis of the FMRl repeat by using flanking primers as described by Pergolizzi et al (1993). Numbers next to pedigree symbols reflect the number of FMRl triplet repeats (Both figures from Warren & Nelson 1994. Used by permission). bility of the premutation alleles, where siblings often have alleles unique from one another as well as distinct from the parent. Analysis by peR is useful for defining accurate premutation repeat lengths and for demonstrating instability, which may be of some utility in genetic counseling (Warren & Nelson 1994), and is useful in situations where limited DNA is a constraint. OTHER TRINUCLEOTIDE REPEAT EXPANSION DISEASES Since the emergence of the trinucleotide repeat expansion mutation responsible for fragile X syndrome, six other diseases caused by trinucleotide expansions Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X S YNDROME 87 Ful Mula' on have been identified (Table 1) (reviewed by Warren & Nelson 199 3, 1994): spinal and bulbar muscular atrophy (SBMA) (La Spada et aI1991); myotonic dystrophy (DM) (Aslanidis et a11992, Brook et a11992, Fu et a11992, Harley et al 1992, Mahadevan et al 1992); Huntington's disease (HD) (Group 199 3); spinal and cerebellar ataxia type 1 (SCA1) (Orr et al 199 3); FraX E mental retardation (Knight et al 1993); and dentatorubral pallidoluysian atrophy (DPA) (Koide et a1 1994, Nagafuchi et aI1994). Interestingly, all trinucleotide repeat diseases described thus far are neurologic diseases, although there is currently no good hypothesis to account for this pattern. Similarities between the trinucleotide repeats of these diseases include that they are all normally 88 Table 1 WARREN & ASHLEY Genetic diseases associated with unstable trinucleotide repeats Change in Repeat number gene Trinucleotide Disease repeat' Normal Fragile X syndrome CGG 5'UTR 6-52 Myotonic dytrophy CTG 3'UTR 5-37 Spinal & bulbar muscular CAG coding Huntington's disease Spinocerebellar ataxia Carrier 50-200 Affected 230 to >1000 function Loss 50 to >1000 mRNA stability 12-34 40-62 Gain CAG coding 11-36 42-100 Gainb CAG coding 19-36 43-81 Gainb CAG coding 7-23 49-75 Gainb CCG- 6-25 Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. atrophy type 1 Dentatorubral pallidoluy- sian atrophy FRAXE mental retardation a All repeat are exonic with the position shown; VTR 116-133 200 to >800 Lossb is untranslated region. b Functional change is not established and is shown based upon similarity to other examples. poly morphic, GC-rich, and expressed sequences, while di f ferences include the exact nucleotide content of the repeat and the location of the repeats within the various messages (Richards & Sutherland 1992). In the majority of these diseases ( HD, SBMA, DPA, and probably SCAl), an unstable CAG repeat within the coding region o f the respective genes undergoes expansion, which produces an a mplified polyglutamine tract in the nascent protein. In the case of X-linked SBMA, Warren & Nelson (199 3) have suggested that the expan sion of the polyglutamine tract in the androgen receptor must constitute a gain of function, perhaps through allowing the receptor to interact with pro moters that it does not normally recognize, because the absence o f mani festations o f testicular fe minization make it unlikely that the receptor is nonfunctional. Although the function o f the gene products in HD and SCAI are currently unknown, the do minant inheritance pattern o f these autosomally inherited disorders argues that expansion o f the polyglutamine tract in these two diseases will constitute a gain-of- function as well. In myotonic dystrophy, a CTG repeat in the 3' untranslated region (UTR) of the myotonin protein kinase gene is enlarged, which results in altered message stability and aberrant intracellular signaling due to abnormal dosage of this protein kinase (Fu et a1 199 3, Sabourin et aI199 3). As previously discussed, expansion o f a CGG repeat in the 5' UTR of the FMRI gene is associated with abnor mal methylation o f the repeat and an upstrea m CpG island leading to transcriptional silencing of FMRI. No infor mation concerning the mechanis m of disease in FraX E mental retardation is currently available because the expanded CGG repeat has not yet been ascribed to a gene. Of all of these repeat expansion mutations, only the repeats of DM and Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X SYNDROME 89 fragile X syndrome, which are within untranslated regions, have full mutations with repeat expansions exceeding several hundred copies. Repeat expansions of the other diseases are usually 2-4 ti mes normal, and maxi mu m repeat sizes rarely exceed 100 copies. This i mplies that the mechanis m of expansion of fragile X syndro me and myotonic dystrophy could be similar, although it is puzzling that DM does not display the parent-of-origin requirement for expan sion observed in fragile X syndrome. However, reductions in trinucleotide repeat sizes, which are almost never seen in fragile X syndrome, are o ften seen in paternal DM alleles (Reyniers et al 1993). Nevertheless, given the fact that the full mutations of DM or fragile X are not a menable to cloning, the other trinucleotide diseases with more subtle trinucleotide expansions will most likely be more infor mative with regard to the mechanis m o f trinucleotide instability. MECHANISM OF THE FRAGILE X REPEAT EXPANSION The molecular mechanis m of trinucleotide repeat expansion still re mains an enigma, although recent advances in the fragile X field have been enlightening. In previous segregation analyses of over 200 fragile X pedigrees, no sponta neous mutations in fe male ga metes causing fragile X syndrome were identified (Sherman et a11984, 1985). Given the fact that fragile X alleles are constantly being re moved from the gene pool because of reduced fitness in affected individuals, Sherman et al (1984, 1985) predicted an extraordinarily high mutation rate in male gametes, on the order of 7.2 x 10-4, to account for the high prevalence of fragile X syndro me within the population. However, sub sequent analyses of fragile X pedigrees have failed to detect any new mutations producing fragile X syndrome (Jacobs et al 1986, Rousseau et al 1991a, Yu et al 199 2, S mits et al 1993). Consistent with the absence of new mutations, S mits et al (1993) recently found that five fragile X males related through co m mon ancestors from the eighteenth century were all concordant for the same allele at the DXS548 locus, a polymorphic marker locus located 15 0 kb proximal to FMRl (Riggins et al 1992). This finding attests to the very old nature of the fragile X mutation in this family and suggests the possibility of linkage disequilibrium in the region enco mpassing the fragile X locus. Haplo type analyses of markers flanking the fragile X locus have, in fact, revealed significant linkage disequilibrium between fragile X syndrome and certain haplotypes, which indicates that a s mall group o f founder chromoso mes, per haps as few as six, might account for the vast majority of fragile X mutations observed today (Richards et al 199 2, Oudet et al 1993). The observation of linkage disequilibrium in the region of the fragile X locus is re miniscent of the situation in individuals with myotonic dystrophy (DM), the gene of which is in co mplete linkage disequilibrium with a single insertion/deletion poly mor- Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 90 WARREN & ASHLEY phis m located 5 kb from the unstable CTG repeat ( I mbert et aI199 3). However, un like the myotonic dystrophy gene, the fragile X locus appears to be in linkage equi libriu m with a discrete subset of hap lotypes, which suggests that multip le mutations of the pri mordia l FMRl repeat are responsib le for the fragile X chro moso mes in today's popu lation (Smits et a l 1993). A low preva lence of new mutations and marked linkage disequi libriu m suggesting highly ancient mutations are unusual findings for X- linked muta tions (Chakravarti 1992), and this scenario is hard to reconcile in light of the high preva lence of fragile X syndrome in the popu lation. One possib le expla nation for this pheno mena is an unexpectedly high nu mber of pre mutations in the popu lation that go undetected in the absence of any obvious phenotype. In favor of this hypothesis, Snow et a l (199 3) found the frequency of premuta tion a l le les in the nor ma l popu lation to be 0.8%. A mu ltistep model has been proposed that invokes a discrete set of mutations of a nor ma l primordial a l le le to a pre-pre mutation, or a predisposed a l le le, that is relatively stab le for many years (thus accounting for the linkage disequilibriu m observed ) but has the potential to convert to unstable premutations that rapidly expand to fu ll mu tations with concurrent disease expression (Morton & Macpherson 1992). In light of this mode l and the above linkage disequi libriu m data, Oudet et a l (199 3) asserted that the most co m mon 30 a l le le of the CGG repeat of FMRl (Brown et al 1993, Snow et al 199 3) was probab ly the pri mordia l a l le le and that larger a l le les in the range of 38-40 repeats represent the predisposed a l le les that u lti mate ly account for a l l of the fragile X chro moso mes. In addition, it has been suggested that a mutation of one of the interspersed AGG trip lets to CGG within the nor ma l ly cryptic repeat of FMRl (Pieretti et a1 1991, Verkerk et a1 1991) could account for the pri mordial mutation event ( Oudet et a1199 3) because repeats that are purer in content are less stable ( Weber 1990 ). Final ly, superimposed upon this mode l is the like lihood of poly merase s lippage during replication of these reiterated sequences ( I mbert et a l 1993), which accounts for the s ma l ler, inert variation in the nor ma l CGG repeat length contributing to the nor mal ly poly morphic nature of the repeats. If this mode l is va lid, then it fol lows that the primary new mutations responsible for the cases of fragile X syndro me today probab ly occurred many generations ago, and any new mutations identified today may not manifest the mselves phenotypica l ly for many years to co me. The apparent dearth of expansion of premutation a l le les into the fu l l muta tion range upon trans mission from carrier males to their obligate carrier daugh ters has also recently been addressed. Upon discovery of a rare mating event by an affected male, Wi l lems et al (1992) fol lowed the segregation of the fragile X mutation from an affected male through his nor ma l carrier daughter to an affected grandson. At the mo lecular level, the daughter received only a premutation a l le le, whereas the grandfather was mosaic for fu l l and premuta- FRAGILE X S YNDROME 91 Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. tion-size alleles, which suggested a priori that the grandfather displayed germ line mosaicis m as well as so matic mosaicism. However, subsequent analyses o f a f fected males with full mutations in so matic tissues revealed only pre muta tion alleles in their sper m (Reyniers et al 1993). One interpretation o f these findings is that partial trinucleotide repeat expansion occurs within the male ga mete, with subsequent selection against sper m with fully expanded repeats. Another interpretation is that trinulceotide repeat expansion occurs postzygoti cally following the sequestering o f primordial ger m cells that are spared the massive expansion. However, a maternal i mprint must be invoked that leads to so matic expansion of maternal pre mutations. With regard to this model o f postzygotic expansion, i t will be crucial t o determine whether oocytes with full mutations are observed in ovaries o f carrier fe males. To date, no such analysis o f repeat sizes in oocytes has been reported. A prediction o f such a postzygotic expansion model is that a variable degree o f repeat length mosaicis m should be present in the so matic tissues o f a f fected patients. Indeed, so matic mosaicism in cells o f fragile X patients has been well documented ( Fu et al 1991, Pieretti et al 1991, Rousseau et al 1991a). In a study o f 511 individuals from 63 fragile X fa milies, Rousseau et al (1991b) reported that approxi mately 15% o f individuals with mutations within the fully expanded range also displayed pre mutation alleles in a subset o f cells. In individuals such as these, the pre mutation allele is predo minately un methylated and transcriptionally active, whereas the full mutation allele is most often fully methylated and transcriptionally silent (Pieretti et al 1991). In analysis o f a 13-week male fetus, nearly identical mosaic patterns were observed across all tissues analyzed, and subsequent prolonged cell culture provided no evidence o f mitotic instability o f the alleles initially present (Wohrle et aI1993). Inter estingly, chorionic villi obtained at 10 weeks gestation for prenatal diagnoses displayed fully expanded, unmethylated FMRI repeats, thereby deli miting the window for trinucleotide repeat expansion to be fore this ti me and methylation o f expanded repeats to sometime afterwards. Thus, many data support the idea that expansion occurs postconceptionally, and o f the two models described, it is the most widely accepted at the present time. One major shortcoming of the postzygotic model of repeat expansion is its inability to satis factorily account for the strict parental influence observed in fragile X pedigrees. I f this model is valid, then an i mprinting phenomenon clearly must also occur to account for the absence of repeat expansion in daughters o f carrier males. As previously mentioned, Laird (1987) has pro posed that fragile X syndro me results fro m the abnor mal persistence o f an i mprint due to inco mplete erasure of X-inactivation on the fragile X chromo some in female gametes. Although the observation of abnormal methylation associated with repeat expansion is consistent with this hypothesis, the occur rence o f mosaicis m for full and pre mutation alleles and the fact that pre muta- 92 WARREN & ASHLEY Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. tion alleles are unmethylated (Pieretti et al 1991) are dif ficult to reconcile with regard to Laird's hypothesis. Further more, corroborating data from two labs indicate that expansion precedes methylation in the embryo (Sutherland et al 1991, Sutclif fe et al 1992), which makes it questionable whether methylation in any form contributes to the lack o f expansion in daughters of carrier males. So, validation o f the postzygotic model o f repeat expansion is incumbent upon identi fication o f the factor responsible for the strict maternal origin o f expanded FMRI alleles. CHARACTERIZATION OF FMRl AND THE GENE PRODUCT Since the identi fication o f the FMRI gene, much has been gleaned with regard to its physical structure and pattern of expression. The FMRI gene includes 17 exons encompassing 38 kb o f the human genome (Eichler et al 1993 ). The eGG repeat resides within the first exon of the gene and is positioned 25 0 basepairs downstream o f the CpG island that is aberrantly methylated in fragile X patients ( Fu et al 1991, Eichler et al 1993 ) and may serve as the pro moter o f the FMRI gene ( Hwu et al 1993 ). On Northern analysis, expression in a nu mber o f human tissues both related and unrelated to the fragile X phenotype is observed; these include brain, testes, placenta, lung, kidney, and heart ( Hinds et al 1993 ). In situ R NA expression studies in adult mouse tissues expanded the list of tissues by adding esophageal epithelium, thymus, spleen, ovary, eye, colon, uterus, thyroid, and liver; expression in heart, aorta, or muscle was not observed in the adult mouse ( Hinds et al 1993 ). In cross sections of mouse brain, Hinds et al (1993 ) reported expression predominately in neurons as opposed to white matter or glial celis, with highest levels o f expression in the cerebellu m, habenula, and the granular layers o f the hippocampus and cerebral cortex. In a recent in situ R NA expression analysis o f cross sections o f brain from a 25-week-old human fetus (Abitbol et a11993), the authors report FMRI expression mainly in neurons; the nucleus basalis magnoceliularis and areas o f the hippocampus are most intense. These results are consistent with the observations by Hinds et al (1993 ) in that predominant labeling is o bserved in areas critical to limbic circuitry, which is a pathway in the brain involved in cognition, memory, and behavior. Im munohistochemical analysis with antibodies against FMRP con firmed the predo minant neuronal and sparse glial distribution o f FMRP previously indi cated by in situ R NA studies (Devys et al 1993 ). In addition, two reports o f intracellular immunolocalization o f FMRP concur that the protein is localized predo minately to the cytosol, although nuclear localization in the a bsence o f cytosolic expression was observed i n a small proportion o f cells (Devys e t al 1993, Verheij et aI1993 ). Interestingly, the a mino ter minal half o f the protein Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X SYNDROME 93 displayed exclusive nuclear localization according to Devys et al (1993), although this half of the protein displays no nuclear localization signal and lacks the putative nuclear localization signal near the carboxy ter minus de scribed by Verkerk et al (1991 ). Thus, it re mains unclear whether FMRP might, under certain conditions, have a functional role in the nucleus. The mouse FMRI ho mologue has been identified and shown to be re mark ably conserved, with nucleotide and amino acid identity values of 95% and 9 7%, respectively, within the coding region (Ashley et al 1993a). The CGG repeat responsible for the fragile X phenotype in hu mans is also conserved in mouse both in its position and nucleotide sequence, although the repeat nu mber is reduced to nine copies (8 CGGs and 1 CGA) (Ashley et al 1 993a). An in-frame stop codon identified upstream of the human repeat and a conserved ATG located 66 and 69 nucleotides downstream of the CGG repeat in mouse and hu man, respectively, delimit the CGG repeat to the 5' untranslated region ( 5' UTR) of the FMRI message and predict a putative protein product of 6 9 kD (Ashley et a l 1993a ). Additionally, alternative splicing of the FMRI gene has been docu mented. The results of this splicing predict at least twelve potential isofor ms of the FMRI predicted protein, FMRP, in all tissues ana lyzed and suggest the potential for intracellular functional diversity of the FMRI gene product (Verkerk et al 1 993, Ashley et al 1 993a). In favor of the notion of functional heterogeneity is the fact that a subset of the alternative splicing events in FMRI alter the normal reading frame and produce isofor ms with novel carboxy ter mini and quite distinct hydropathy profiles (Ashley et al 1993a). Two copies of a 30-a mino acid do main conserved across evolution fro m bac teri a to humans have been identified within the amino terminal half of the FMRP predicted sequence (Ashley et al 1993b, Siomi et al 1993b). This repeated do main, termed a KH do main (Siomi et al 1993a ), is also present in a nu mber of proteins that have an interaction with R NA in co m mon, including one member of the hnRNP class of ribonucleoproteins, hnRNP K. Also iden ti fied within the carboxy ter minal portion FMRP a mino acid sequence are two copies of the RGG box (arginine-glycine-glycine) (Ashley et al 1 993b), an a mino acid motif i mplicated in both R NA as well as DNA binding (Christensen & Fuxa 1988). Nucleic acid binding assays of in vitro translated FMRP reveal that it is an R NA binding protein that binds to its own R NA transcript with high affinity (Kd 5.7 nM) and to a lesser extent to a pool of R NAs from human fetal brain; weak binding of FMRP to both single-strand and double strand DNA is observed as well (Ashley et al 1 993b). Selective binding of FMRP to individual human fetal brain transcripts occurs, and it is suggested that approxi mately 4% of the messages present in human fetal brain might be bound by FMRP, which is consistent with the highly pleiotropic nature of the fragile X phenotype (Ashley et al 1993b ). Finally, a 2:1 stoichiometry of = 94 WARREN & ASHLEY binding of R NA:protein is reported, which i mplies that each protein molecule has the potential to interact with two R NA molecules (Ashley et al 1993b). Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. CONCLUSIONS Fragile X syndrome accounts for a major proportion of mental retardation in hu mans. In addition to the intellectual deficit, these patients also exhibit a wide range of behavioral problems, including autistic-like features. Thus, the mo lecular understanding of this syndrome should lead to further understanding of the higher cognitive functioning in hu mans. Indeed, much has already been learned from the cloning of the fragile X gene, FMR1. The mutational basis of the syndro me is the extraordinary expansion of a trinucleotide repeat within the 5' untranslated region of the gene. Since this discovery, six other genetic diseases, all neurological in nature, have now been attributed to unstable triplet repeats within exons of different genes. This novel mutational mechanis m will likely be found responsible for even more genetic disorders. The mechanis m of repeat expansion is poorly understood and is a fertile and i mportant area of inquiry. When the CGG repeat in the FMRl gene exceeds 23 0 repeats, the gene is heavily methylated and transcriptionally suppressed. The absence of the en coded protein, FMRP, is therefore believed responsible for the mental retar dation and associated phenotype. This cytosolic protein has been de monstrated to be a selective R NA-binding protein, interacting with as much as 4% of human brain mRNA. Thus, the further understanding of this interaction and the identification of those genes whose messages interact could lead to a great deal of insight into the mechanis ms of cognitive function in hu mans. ACKNOWLEDGMENTS STW is an investigator with the Howard Hughes Medical Institute and CTA is a predoctoral fellow with the March of Dimes Birth Defects Foundation. Any Annual Review chap ter, as well as any article cited in an Annual Review chapter, may be p urchased from the AnnulIl Reviews Preprints lind Reprints service. 1-800-347-8007; 415-259-5017; email: [email protected] Literature Cited Abitbol M, Menini C, Delezoide A-L, Rhyner T, Vekemans M, Mallet 1. 1993. Nucleus basalis magnolaris and hippocampus are the major sites of FMR-l expression in the human fetal brain. Nature Genet. 4:147-52 American Psychiatric Association. 1987. Diag nostic and Statistical Manual of Mental Dis- orders, pp. 28-33. Washington DC: Am. Psychiatr. Assoc. 567 pp. 3rd ed. Ashley CT, Sutcliffe IS, Kunst CB, Leiner HA, Eichler EE, et al. 1993a. Human and murine FMR-l: alternative splicing and translational initiation downstream of the CGG-repeat. Nature Genet. 4:244-51 Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. FRAGILE X SYNDROME Ashley CT, Wilkinson KD, Reines D, Warren ST. 1993b. FMRI protein: conserved RNP family domains and selective RNA binding. Science 262:563--66 Aslanidis C, Jansen G, Amemiya C, Shutler G, Mahadevan M, et al. 1992. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature 355:548-51 Bell MV, Hirst MC, Nakahori Y, MacKinnon RN, Roche A, et al. 1991. Physical mapping across the fragile X: hypermethylation and clinical expression of the fragile X syndrome. Cell 64:861-66 Biederman J, Munir K, Knee D, Armentano M, Autor S, et al. 1987. High rate of affective disorders in probands with attention deficit disorders and their relatives-a controlled family study. Am. 1. Psychiatry 144:330-33 Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, et al. 1992. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 68:799-808 Brown WT, Houck GE, Jeziorowska A, Levin son FN, Ding X, et al. 1993. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 270(13):1569-75 Brown WT, Jenkins EC. 1992. The fragile X syndrome. Mol. Genet. Med. 2:39-65 Brown WT' Jenkins EC, Friedman E, Brooks J, Wisniewski K, et al. 1982. Autism is as sociated with fragile X syndrome. 1. Autism Dev. Dis. 12:303-8 Butler MG, Allen A, Haynes JL, Singh DN, Watson MS, Breg WR. 1991a. Anthropomet ric comparison of mentally retarded males with and without the fragile X syndrome. Am. 1. Med. Genet. 38:260-68 Butler MG, Brunschwig A, Miller LK, Hager man RJ. 1992. Standards for selected an thropometric measurements in males with the fragile X syndrome. Pediatrics 89: 105962 Butler MG, Mangrum T, Gupta R, Singh DN. 1991b. A 1 5-item checklist for screening mentally retarded males for the fragile X syn drome. Clin. Gene t. 39 : 347-54 Chakravarti A. 1992. Fragile X founder effect. Nature Genet. 1:237-38 Christensen ME, Fuxa KP. 1988. The nucleolar protein, B-36, contains a glycine and dimeth ylarginine-rich sequence conserved in sev eral other nuclear RNA-binding proteins. Biochem. Biophys. Res. Comm. 155(3): 1278-83 De Boulle K, Verkerk AJMH, Reyniers E, Vits L, Hendrich J, et al. 1993. A point mutation in the FMR-l gene associated with fragile X mental retardation. Nature Genet. 3(1): 3135 95 Devys D, Lutz Y, Rouyer N, Bellocq J-P, Man del J-L. 1993. The FMR-l protein is cyto plasmic, most abundant in neurons, and appears normal in carriers of the fragile X premutation. Nature Genet. 4:335-40 Dietrich A, Kioschis P, Monaco AP, Gross B, Kom B, et aI. 1991. Molecular cloning and analysis of the fragile X region in man. Nucl. Acids Res. 19:2567-72 Dunn HG, Renpenning H, Gerrard JW, Miller JR, Tabata T, Federoff S. 1963. Mental re tardation as a sex-linked defect. Am. 1. Men tal De! 67:827-48 Eichler EE, Richards S, Gibbs RA, Nelson DL. 1993. Fine structure of the human FMRI gene. Hum. Mol. Genet. 2:1 147-53 Filippi G, Rinaldi A, Archidiacono N, Rocchi M, Balasz I, Sinas-Calco M. 1983. Brief re port: linkage between G6PD and fragile X syndrome. Am. 1. Med. Genet. 15: 1 1 3-19 Fisch GS. 1993. What is associated with the fragile X syndrome? Am. 1. Med. Genet. 48(2):112-2 1 Freund LS, Reiss AL, Abrams MT. 1993. Psy chiatric disorders associated with fragile X in the young female. Pediatrics 91:321-29 Froster-Iskenius U, McGillivray BC, Dill FJ, Hall JG, Herbst DS. 1986. Normal male car riers in the fra(X) form of X-linked mental retardation (Martin-Bell syndrome). Am. J. Med. Genet 232:619-32 Fu Y-H, Friedman DI, Richards S, Pearlman JA, Gibbs RA, et al. 1993. Decreased expres sion of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science 260:235-38 Fu Y-H, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, et al. 1991. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67:1047-58 Fu Y-H, Pizzuti A, Fenwock RG, King J, Rajnarayan S, et al. 1992. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255 : 1 256-58 Gideon AK, Baker E, Robinson H, Partington MW, Gross B, et al. 1992. Fragile X syn drome without CCG amplification has an FMRI deletion. Nature Genet. 1:341-44 Glover TW. 1981. FUdr induction of the X chromosome fragile site: evidence for the mechanism of folic acid and thymidine de privation. Am. J. Hum. Genet. 33:234-42 Goodfellow PN, Davies KE, Ropers HH. 1985. Report of the committee on the genetic con stitution of the X and Y chromosomes. Cytogenet. Cell. Genet. 40:296-352 Group THDCR. 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromo somes. Cell 72:971-83 Gustavson K-H, Blomquist H, Holmgren G. 1986. Prevalence of fragile-X syndrome in Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 96 WARREN & ASHLEY mentally retarded children in a Swedish county. Am. J. Med. Genet 23:58 1 -88 Hagerman RJ. 1991. Physical and behavioral phenotype. In Fragile X Syndrome, Diagno sis, Treatment and Research, ed. RJ Hager man, AC Silverman, pp. 3-68. Baltimore: Johns Hopkins Univ. Press Hagerman RJ, Amiri K, Cronister A. 1 99 1 . Fragile X checklist. Am. J. Med. Genet 38: 283-87 Hagerman RJ, Jackson C, Amiri K, Silverman AC, OConner R, Sobensky W. 1 992. Girls with fragile X syndrome: physical and neu rocognative status and outcome. Pediatrics 89(3):395-400 Hagerman RJ, Sobensky WE. 1989. Psychopa thology in fragile X syndrome. Am. J. Ortho psychiatry 59: 142-52 Hagerman RJ, Synhorst DP. 1984. Mitral valve prolapse and aortic dilitation in fragile X syn drome. Am. J. Med. Genet 1 7 : 1 23-31 Hagerman RJ, Van Housen K, Smith ACM, McGavran L. 1984. Consideration of connec tive tissue dysfunction in fragile X syndrome. Am. J. Med. Genet 17 : 1 1 1-2 1 Hansen RS, Gartler SM, Scott CR, Chen SoH, Laird CD. 1 992. Methylation analysis of CGG sites in the CpG island of the human FMRI gene. Hum. Mol. Genet. 1 (8):571-78 Harley HG, Brook JD, Rundle SA, Crow S, Reardon W, et a!. 1992. Expansion of an unstable DNA region and phenotypic varia tion in myotonic dystrophy. Nature 355:54546 Harper PS. 1989. Myotonic dystrophy and re lated disorders. In Principles and Practice of Medical Genetics, ed. AEH Emery, DL Rimoin, pp. 579-97. Philadelphia: Saunders. 894 pp. Harrison CJ, Jack EM, Allen TD, Harris R. 1983. The fragile X: a scanning electron microscope study. J. Med. Genet. 20:28085 Heitz D, Rousseau F, Devys D, Saccone S, Abderrahim H, et a!. 1 99 1 . Isolation of se quences that span the fragile X and identifi cation of a fragile X-related CpG island. Science 25 1 : 1 236-39 Hinds HL, Ashley CT, Nelson DL, Warren ST, Housman DE, Schalling M. 1993. Tissue specific expression of FMR 1 provides evi dence for a functional role in fragile X syn drome. Nature Genet. 3:36-43 Hirst MC, Roche A, Flint n, MacKinnon RN, Bassett JHD, et al. 199 1 . Linear order of new and established markers around the fragile site at Xq27.3. Genomics 10:243-49 Homstra IK, Nelson DL, Warren ST, Yang TP. 1993. High resolution methylation analysis of the FMR 1 gene trinucleotide repeat region in fragile X syndrome. Hum. Mol. Genet. 2(10) : 1 659-65 Howard-Peebles PN, Friedman JM. 1985. Un- affected carrier males in families with fragile X syndrome. Am. J. Hum. Genet. 37:956-64 Hwu W-L, Lee YoM, Lee SoC, Wang T-R. 1993. In vitro DNA methylation inhibits FMRI promoter. Biochem. Biophys. Res. Commun. 193:324-29 Imbert G, Kretz C, Johnson K, Mandel J-L. 1993. Origin of the expansion mutation in myotonic dystrophy. Nature Genet. 4:72-76 Jacobs PA, Bullman H, Macpherson J, Youings S, Rooney V, et a!. 1993. Population studies of the fragile X: a molecular approach. J. Med. Genet. 30:454-5 9 Jacobs PA, Sherman S, Turner G, Webb T. 1986. The fragile (X) syndrome: the mutation problem. Am. J. Med. Genet 23:61 1-17 Jenkins ED, Brooks J, Duncan CJ, Sanz MM, Silverman WP, et a!. 1986. Low frequencies of apparently fragile X chromosomes in nor mal control cultures: a possible explanation. Exp. Cell Bioi. 54:40-48 Knight SJL, Flannery A V, Hirst MC, Campbell L, Christodoulou Z, et al. 1993. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell 74: 1 27-34 Koide R, Ikeuchi T, Onodera 0, Tanaka H, Igarashi S, et al. 1 994. Unstable expansion of CAG repeat in hereditary dentatorubral pallidoluysian atrophy (DRPLA). Nature Genet. 1 :9-13 Kremer EJ, Pritchard M , Lynch M, Yu S , Hol man K, et a!. 1991a. Mapping of DNA insta bility at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science 252: 1 7 1 1-14 Kremer EJ, Yu S, Pritchard M, Nagaraja R, Heitz D, et a!. 1991b. Isolation of a human DNA sequence which spans the fragile X. Am. J. Hum. Genet. 49:656-61 La Spada AR, Wilson EM, Lubahn DB, Har ding AE, Fischbeck KH. 1 99 1 . Androgen re ceptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352:77-79 Laird CD. 1987. Proposed mechanism of inher itance and expression of the human fragile X syndrome of mental retardation. Genetics 1 1 7:587-99 Loehr JP, Synhorst DP, Wolfe RR, Hagerman RJ. 1986. Aortic root dilatation and mitral valve prolapse in the fragile-X syndrome. Am. J. Med. Genet. 23:1 89-94 Loesch DZ, Huggins R, Hay DA, Gedeon AK, Mulley Ie, Sutherland GR. 1993. Genotype phenotype relationships in fragile X syn drome: a family study. Am. J. Hum. Genet. 5 3 : 1 064-73 Lubs JA Jr. 1 969. A marker X chromosome. Am. J. Hum. Genet. 2 1 : 23 1-44 Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, et a!. 1992. Myotonic dys trophy mutation: an unstable CTG repeat in the 3 untranslated region of the gene. Sci ence 255 : 1 253-55 FRAGILE X SYNDROME Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. Martin JP, Bell J. 1943. A pedigree of mental defect showing sex linkage. J. Neurol. Neu rosurg. Psychol. 6: 1 54-57 McConkie-Rosell A, Lachiewicz AM, Spiri digliozzi GA, Tarleton J, Schoenwald S, et al. 1 993. Evidence that methylation of the FMR-1 1ocus is responsible for variable phe notype expression of the fragile X syndrome. Am. J. Hum. Genet. 53:800-9 McKusick V A, Francomano CA, Antonarakis SE, eds. 1992. Mendelian Inheritance in Man: Catalogs ofAutosomal Dominant, Au tosomal Recessive, and X-linked Phenotypes. Baltimore: John Hopkins Univ. Press. 2028 pp. 1 0th ed. Morton NE, Macpherso, IN. 1 992. Population genetics of the fragile X syndrome: multialle lic model for the FMRI locus. Proc. Natl. Acad. Sci. USA 89:4215-17 Nagafuchi S, Yanagisawa H, Sato K, Shirayama T, Ohsaki E, et a1. 1 994. Denta torubral pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromo some 12p. Nature Genet. 1 : 1 4- 1 8 Neri G, Chiurazzi P , Arena F , Lubs H A , Glass IA. 1992. XLMR genes: update 1992. Am. J. Med. Genet. 43:373-82 Oberle I, Heilig R, Moisan JP, Kloepfer C. 1986. Fragile-X mental retardation syndrome with two flanking polymorphic DNA mark ers. Proc. Natl. Acad. Sci. USA 83: 1 0 1 6-20 Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, et a1. 1 99 1 . Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252: 1097-102 Opitz JM. 1986. On the gates of hell and a most unusual gene. Am. J. Med. Genet. 23:1-10 (erratum 1987. 26:37) Opitz JM, Sutherland GR. 1984. International workshop on the fragile X and X-linked men tal retardation. Am. J. Med. Genet. 17:5-94 Opitz 1M, Westphal 1, Daniel A. 1984. Discov ery of a connective tissue dysplasia in the Martin-Bell syndrome. Am. J. Med. Genet. 1 7 : 101-9 Orr HT, Chung M, Banfi S, Kwiatkowski TJ. Servadio A, et al. 1993. Expansion of an unstable trinucleotide (CAG) repeat in spino cerebellar ataxia type 1 . Nature Genet. 4: 221-26 Oudet C, Mornet E, Serre lL, Thomas F. Lentes-Zengerling SL, et al. 1993. Linkage disequilibrium between the fragile X muta tion and two closely linked CA repeats sug gests that fragile X chromosomes are deri ved from a small number of founder chromo somes. Am. J. Hum. Genet. 52:297-304 Pai GS, Sprenkle lA, Do IT, Mareni CE, Mi geon BR. 1980. Localization of loci for hy poxanthine phosphoribosyltransferase and glucose-6-phosphate dehydrogenase and bio chemical evidence of nonrandom X chromo some expression from studies of a human 97 X-autosome translocation. Proc. Natl. Acad. Sci. USA 77(5):2810-13 Pembrey ME, Winter RM, Davies KE. 1 985. A premutation that generates a defect at cross ing over explains the inheritance pattern of fragile X mental retardation. Am. J. Med. Genet. 2 1 :709-17 Penrose LS. 1938. A Clinical and Genetic Study of 1,280 Cases of Mental Defect. London: MRC Pergolizzi RG, Erster SH, Goonewardena P, Brown WT. 1 992. Detection of full fragile X mutations by polymerase chain reaction. lAncet 339:27 1 -72 Pieretti M, Zhang F, Fu YH, Warren ST, Oostra BA, et ai. 199 1 . Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66: 8 1 7-22 Popper CWo 1988. Disorders usually first evi dent in infancy, childhood, or adolescence. In Textbook ofPsychiatry, ed. JA Talbot, RE Hales, SC Yudofsky, pp. 649-735. Washing ton, D.C: Am. Psychiatr. Reiss AL, Feinstein C, Toomey KE, Goldsmith B, Rosenbaum K, Caruso MA. 1986. Psychi atric disability associated with the fragile X syndrome. Am. J. Med. Genet. 23:393-401 Reiss AL, Freund L. 1 992. Behavioral pheno type of fragile X syndrome. Am. J. Med. Genet. 43:35-46 Reiss AL, Hagerman Rl, Vingradov S, Abrams M, King RJ. 1988. Psychiatric disability in carrier females of the fragile X syndrome. Arch. Gen. Psychiatr. 45:25-30 Reyniers E, Vits L, De Boulle K, Van Roy B , Van Velzen D, e t al. 1993. The full mutation in the FMR-l gene of male fragile X patients is absent in their sperm. Nature Genet. 4: 143-46 Richards RI, Holman K, Friend K, Kremer E, Hillen D, et al. 1 992. Evidence of founder chromosomes in fragile X syndrome. Nature Genet. 1 :257-60 Richards RI, Sutherland GR. 1992. Heritable unstable DNA sequences. Nature Genet. I : 7-9 Riggins Gl, Sherman SL, Oostra BA, Sutcliffe IS, Feitell D, et al. 1992. Characterization of a highly polymorphic dinucleotide repeat 150 kb proximal to the fragile X site. Am. J. Med. Genet. 43:237-43 Rousseau F, Heitz D, Biancalana V, Blu menfield S, Kretz C, et al. 1991a. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N. Engl. J. Med. 325 : 1 673-81 Rousseau F, Vincent A, Rivella S, Heitz D, Triboli C, et al. 1991b. Four chromosomal breakpoints and four new probes mark out a l O-cM region encompassing the fragile-X locus (FRAXA). Am. J. Hum. Genet. 48: 108-16 Sabourin LA, Mahadevan MS, Narang M, Lee 98 WARREN & ASHLEY Dse, Surh LC, Komeluk R G. 1993. Effect of the myotonic dystrophy (DM) mutation on mRNA levels of the DM gene. Nature Genet. Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. 4(3):233-38 Schwartz CEo 1993. Invited editorial: X-linked mental retardation: in pursuit of a gene map. Am. J. Hum. Genet. 52: 1025-3 1 Sherman SL, Jacobs PA. Morton NE, Froster Iskenius V. Howard-Peebles PN. et al . 1985. Further segregation analysis of the frag ile X syndrome with special reference to transmit ting males. Hum. Genet. 69:289-99 Sherman SL. Morton NE. Jacobs PA. Turner G. 1 984. The marker (X) syndrome: a cyto genetic and genetic analysis. Ann. Hum. Genet. 48:21-37 Siomi H, Matunis MJ. Michael WM. Dreyfuss G. 1 993a. The pre-mRNA bi nding K protein contains a n ovel evolutionarily conserved motif. Nud. Acids Res. 21 (5): 1 1 93-98 Siomi H. Si omi MC, Nussbaum RL, Dreyfuss G. 1993b. The protein product of the fragile X gene, FMR 1 . has characteristics of an RNA binding protein. Cell 74:29 1 -98 Smith SD, Kimberling WJ. Pennington SF, Lubs HA. 1 983. Specific reading disability identification of an inherited fonn through linkage analysis. Science 219:1 345-47 Smits APT, Dreesen JCFM, Post JG, Smeets DFCM, de Die-Smulders C, et a!. 1993. The fragile X syndrome: no evidence for any re cent mutati ons . l. Med. Genet. 30:94-96 Snow K, Doud LK. Hagennan R, Pergolizzi RG, Erster SH, Thibodeau SN. 1993. A nal ysis of a CGG sequence at the FMR-l l ocus in fragile X families and in the general pop ulation. Am. J. Hum. Genet. 5 3 : 1 2 1 7-28 Sutcliffe JS. Nelson DL, Zhang F, Pi erelti M , Caskey CT, et a ! . 1 992. DNA methyl ation represses FMR-l transcription in fragile X syndrome. Hum. Mol. Genet. 1 :397-400 Sutherland GR. 1 977. Fragile sites on human chromosomes; demonstration of the ir depen dence on the type of tissue culture medium. Science 197:265-66 Sutherland GR, Gedeon A, Komman L, Donnelly A. Byard RW. et a!. 1 99 1 . Prenatal diagnosis of fragile X syndro me by direct detection of the unstable DNA sequence. N. Engl. J. Med. 325:1 720-22 Sutherland GR. Hecht F, M ulley JC, Glover TW, Hech t BK. 1985. Fragile Sites 011 Humall Chromosomes. New York: Oxford Univ. Pre�s. 280 pp. Suthers GK. Hyland VJ, Callen DF, OberIe I, Rocchi M, et al. 1 990. Physical mappi ng of new DNA probes near the fragile X mutation (FRAXA) by using a panel of ce l l lines. Am. J. Hum. Genet. 47: 1 87-95 Suthers GK, Mulley JC . Voelckel MA, Dahl N. Vaisanen ML, et al. 1 991 . Linkage heteroge n eity near the fragile X locus in normal and fragile X families. Genomies 1 0:576--82 Tarleton J, Richie RX, Schwartz C, Rao K, Aylsworth AS. Lachi ewi cz A. 1 993. An ex tensive de novo deletion removing FMRI jn a patient with mental retardation and the fragile X syn drome phenotype. Hum. Mol. Genet. 2{U):1973-74 Taylor A, Safanda IF. Fall MZ, Quince C, Lang KA, et al. 1 994. Molecular predictors of cog n itive involvement in female carriers of frag ile X syn drome . lAMA 271 (7):507-14 Verheij e, Bakker CE, deGraff E, Keulemans J, Wil lemson R, et a1. 1993. Characterization and localization of the FMR -I gene product associated wi th fragile X syndrome. NaTUre 363:722-24 Verkerk AJMH, de Graff E, De BoulIe K, Ei chler EE, Konecki DS, et al. 1993. Alter native splicing in the fragile X gene FMRI . Hum. Mol. Genet. 2(4):399-404 Verkerk AJMH, Pieretti M. Sutcliffe JS, Pu YH, Kuhl DPA. et al. 1 99 1 . Identification of a gene (FMR-l) contai n i ng a eGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syn drome. Cell 65:905-14 Vincent A, Heitz D, Petit C. Kretz C, Oberl I. Mandel JL. 1 99 1 . Abnormal pattern detected in fragile-X patients by pulsed-field gel elec t rophore sis. Nature 349:624-26 Warren ST. Davidson RL. 1984. Expression of fragile X chromosome in human-rodent so matic cell hybrids, Somal. Cell Mol. Genet. 1 0:409- 1 3 Warren ST, Knight SJL, Peters JF. Stayton CL, Consalez GG, Zhang F. 1 990. Isolation of the human chromosomal band Xq28 within so mati c cell hybrids by fragile X site cleavage. Proc. Natl. Acad. Sci. USA 87:3856-60 Warren ST, Nelson DL. 1 993. Trinucleotide repeat expansions i n neurologic disease . Curro Opin. Neurobiol. 3:752-59 Warren ST, Nelson DL. 1 994. Advances in mo lecular analysis of fragile X syndrome . lAMA 27 1 :536-42 Warren ST, Zhallg F, Licameli GR, Peters JF. 1987. The fragile X site in somatic cell hy brids : an approach for molecular cloning of fragile sites. Science 237:420-23 Webb TP. Bundey SE, Thake AI, Todd J. 1 986. Population incidence and segregation ratios in Martin-Bell syndrome. Am. J. Med. Gellet. 23:573-80 Weber lL. 1 990. Infonnativeness of human (dC-dA)n • (dG.dT) polymorphisms. Geno mies 7 :524-30 Willems PJ, Van Roy B, De Boulle K, Vi ts L, Reyn icrs E, et a1. 1 992. Segregation of the fragile X mutation from an affected male to his normal daughter. Hum. Mol. Gellet. J (7): 5 1 1-17 Wohrle D, Hennig I, Vogel W, Steinbach P. 1 993. Mitotic stability of fragile X mutati ons in differentiated cells indic ates early post- FRAGILE X SYNDROME conceptional trinucleotide repeat expansion. Nature Genet. 4:140-42 Annu. Rev. Neurosci. 1995.18:77-99. Downloaded from arjournals.annualreviews.org by EMORY UNIVERSITY on 01/04/10. For personal use only. Wohrle D, Kotzot D, Hirst Me, Manca A, Korn B, et al. 1992. A microdeletion of less than 250 kb, including the proximal part of the FMR-l gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syn drome. Am. 1. Hum. Genet. 5 1 :299-306 99 Yu S , Mulley J, Loesch D, Turner G, Donnelly A, et al. 1992. Fragile-X syndrome: unique genetics of the heritable unstable element. Am. J. Hum. Genet. 50:968-80 Yu S, Pritchard M, Kremer E, Lynch M, Nan carrow J, et al. 1 99 1 . Fragile X genotype characterized by an unstable region of DNA. Science 252: 1 1 79-81